Avoiding corrosion due to humidity
Due to its rapid cooling in the immediate vicinity of cooler surfaces, the ambient air in a room very quickly reaches its dew point, i.e. the state in which the air is already 100% saturated with water vapour and therefore cannot absorb any more water molecules. This is because air at higher temperatures can absorb more moisture.
As a result, the excess moisture condenses into
fine water droplets, which then visibly precipitate onto the cooler surfaces.
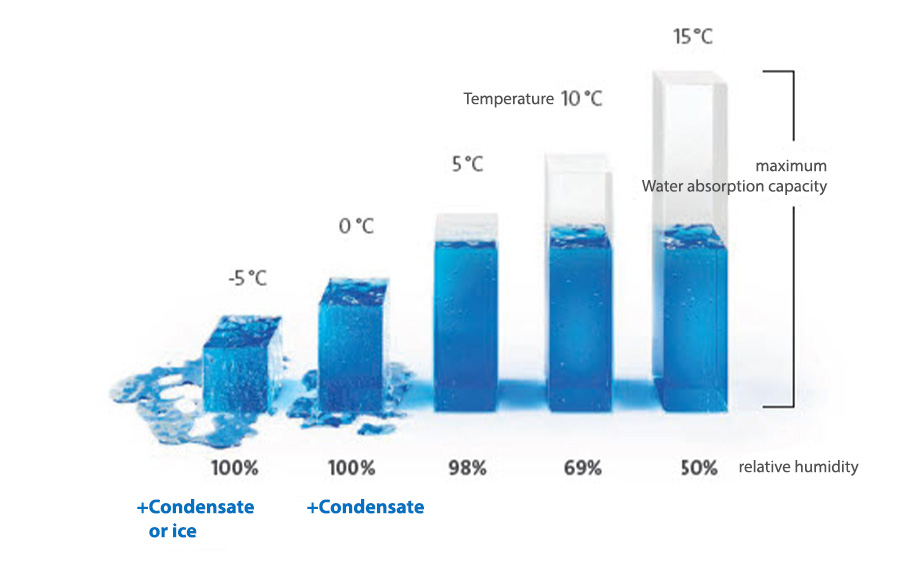
Condensation in production
Even in modern production halls and factory buildings that are designed to be highly airtight, the inflow of warm, humid air from outside cannot be completely avoided, especially in the summer months. This air then condenses on non-insulated pipelines carrying cold media or on cold tanks and containers, metal products or machine components. A thin film of moisture forms on these surfaces.
The problem is that condensation often accumulates unnoticed in hard-to-reach places and forms an ideal breeding ground for mould and other microorganisms. These are then usually very costly to remove. In hygiene-sensitive industries such as food or pharmaceutical production, this can in severe cases lead to anything from operational interruptions to the closure of a business. If condensation drips onto the floor, this increases the risk of accidents to operating personnel from slipping on wet walkways. Moisture can also cause malfunctions in electrical components such as switchgears, sensors or controls.
From condensation to corrosion
Aside from the fact that manual or mechanical drying in industrial infrastructure usually entails considerable effort, time and cost, condensation on metal and steel surfaces also causes them
to undergo an inconvenient change: rusting.
From a chemical point of view, what happens to the affected surfaces is that they oxidize in combination with water and oxygen. This ongoing decomposition process is called atmospheric corrosion. It is the most common type of corrosion in building services and production facilities, although there are many others based on different chemical, physical or biological processes.
The basic conditions for atmospheric corrosion are a relative humidity of 40 percent or above and temperatures greater than 0°C. When the relative humidity is more than 60 per cent, significant corrosion should be expected. The rate of corrosion depends on the duration of humidification and the pH value of the moisture film. Chemical substances such as sulphur, ozone or salts contained in the ambient air in the room or in the incoming air from outside accelerate these reactions immensely in some cases, so that corrosion can start even at lower levels of relative humidity. Even stainless steel pipes can corrode under those circumstances.
Corrosion of steel on a drop of water
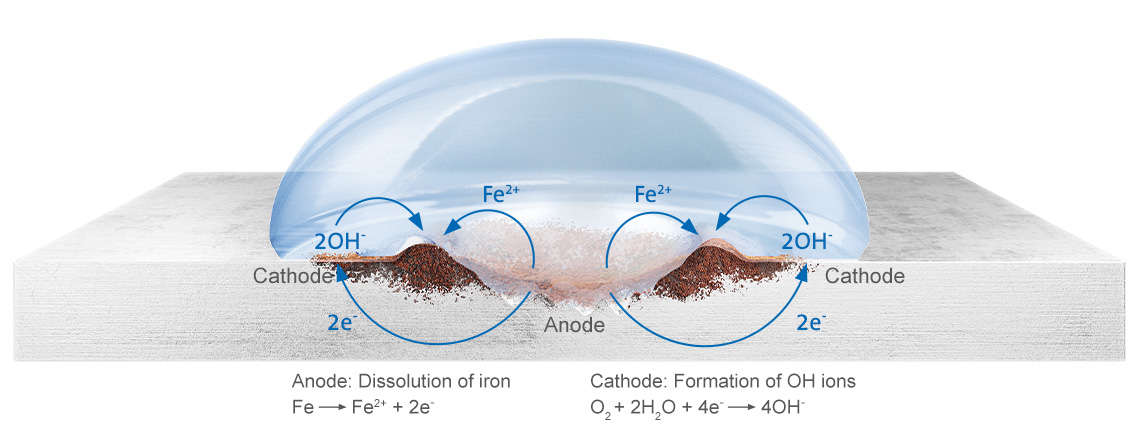
Negative effects of corrosion
Corrosion causes changes in material properties, surface structure and static conditions. These result in functional impairments of a component, material or workpiece, potentially to the point of collapse. In addition, corrosion-related damage can cause production stoppages and safety problems, resulting in significant remediation costs.
According to the Gesellschaft für Korrosionsschutz (Society for Corrosion Protection), the total economic damage caused by corrosion in an industrialized country like Germany amounts to three to four percent of gross national product. This means that between 110 and 140 billion euros were lost due to corrosion damage in 2019 in Germany alone. Corrosion destroys valuable resources and is also often associated with high follow-on costs for the industry. In light of all this, corrosion protection concepts represent an enormous economic factor.
Anti-corrosion measures
It is advisable to apply corrosion-inhibiting protective coatings. However, these coatings must be applied with extreme care to a properly pre-treated surface. If this is not done adequately, the water vapour contained in the air will still diffuse onto the metal surface, meaning that corrosion will take place sooner or later. One visible sign of this is flaking of the protective coating.
It is better to keep all components and surfaces that are at risk of corrosion dry at all times. Short of lowering the humidity in the room in general, one possibility here is to use what are known as demand controlled dehumidification systems, which automatically adjust their dehumidification level according to the existing moisture load. This is done using a dew point sensor mounted directly on a part of the building or system where the air is at risk of falling below the dew point. It measures the relative humidity directly on its surface.
The air dehumidification system only starts up if there is an imminent danger of the dew point falling below the limit and condensation forming on the surface to be protected. This is a particularly energy efficient solution even in rooms where the ambient humidity can fluctuate, as the operating times and thus the energy consumption of the air dehumidifier are reduced to an absolute minimum here.





