Introduction
Most respiratory viruses show a pronounced seasonality, but this has yet to be documented for SARS-CoV-2.
METHODS: We studied the course of COVID-19 in 6,914 patients admitted to hospitals in Europe and China. In addition, we evaluated the course of the disease symptoms in 37,187 people who reported symptoms in the COVID Symptom Study app.
Findings: A meta-analysis of the mortality risk in seven European hospitals estimated the odds per extension of the admission date by 1 day to 0.981 (0.973-0.988, < p 0.001) and per increase in ambient temperature by 1 ° C to 0.854 (0.773-0.944, p = 0.007). Statistically significant decreases of comparable magnitude in the mean length of stay in hospital, the likelihood of being transferred to the ICU, and the need for mechanical ventilation were also observed in most, but not all, hospitals. Analysis of the individually reported symptoms of 37,187 people in the UK also showed the decrease in symptom duration and disease severity over time.
Interpretation: The severity of COVID-19 in Europe decreased significantly between March and May and the seasonality of COVID-19 is the most likely explanation.
Background
More than a million COVID-19-related deaths had been reported as of October 1, 2020, but a significant number of those infected with SARS-CoV-2 (over 80% in some populations) manage to contain the upper respiratory tract infection and develops no visible symptoms despite PCR-positive viral RNA (1). So far, little attention has been paid to the effects of environmental conditions on the individual course of diseases.
The first study of the environmental effects on COVID-19 infection rate in 30 Chinese provinces found significant negative associations with temperature and relative humidity in Hubei province with a decrease in cases of 36-57% for every 1 ◦C and 11- 22% for every 1% increase in relative humidity; these relationships were not uniform in other provinces (2). Negative effects on COVID-19 transmission at warmer temperatures were also observed in Turkey (3), Mexico (4), Brazil (5), and United States (6), while a similar association with humidity was reported in Australia but temperature had no effect on virus transmission (7). The study from Brazil observed a flattening in temperature effects on virus transmission at 25.8 ◦C, suggesting that warmer weather does not lead to a decrease in transmission, which is in line with studies from Iran and Spain, where no changes in transmission rates were observed at different temperatures and humidity (8, 9). These studies are contradicting and give no clear indication whether there is a relationship between temperature, humidity and virus transmission. The global view seems to provide a clearer conclusion; all three studies that performed an analysis on a global scale found a relationship between higher humidity, warmer temperatures and a lower transmission rate (10). However, climate-dependent epidemic modeling suggested that the population's lack of immunity is a much stronger factor in virus transmission and that summer temperatures will not significantly limit the spread of the COVID-19 pandemic (11). This is in line with the high number of people infected in tropical countries and the increase in cases in the southern United States in the second half of June 2020.
Recent studies report an increasing number of SARS-Cov-2-positive asymptomatic people (1), but it is not clear whether the apparent increase in people with mild or no symptoms is due to the changed scope of the tests or to some other property of the SARS-CoV-2 virus. With the aim of investigating the relationship between humidity and ambient temperature and the severity of the COVID-19 disease, we analyzed the individual patient data of 6,914 patients with COVID-19 who had been in hospitals in Bergamo (Italy), Barcelona ( Spain), Coburg (Germany), Helsinki (Finland), Milan (Italy), Nottingham (Great Britain), Warsaw (Poland), Zagreb (Croatia) and the province of Zhejiang (China) and compared them with the ambient temperature and the calculated indoor humidity. We also analyzed information on the severity of COVID-19 from the COVID Symptom Study app, which collects information from 37,187 people in the UK.
Methods
Cohorts studied
We collected information on hospital admission, discharge dates, intensive care unit (ICU) admission, need for mechanical ventilation, and type of discharge (live or dead) for 5,229 consecutive patients diagnosed with COVID-19 in six European hospitals and 13 hospitals in Zhejiang Province, China have been hospitalized since the pandemic began (Table 1). We included patients with a confirmed diagnosis of COVID-19 at the time of admission. We confirmed that patients had a positive result on the polymerase chain reaction test of a nasopharyngeal specimen and / or a clinical / radiological diagnosis of COVID-19. Patients were not followed up after discharge, but early readmissions related to COVID-19 were considered as part of the COVID-19 history. The study protocol complied with the ethical guidelines of the Declaration of Helsinki of 1975. In the hospitals in Zhejiang, the ASST Papa Giovanni XXIII Hospital in Bergamo, the Hospital del Mar in Barcelona and the University Hospital Helsinki, the local ethics committees approved this retrospective study with COVID-19- Patient data. The ethics committee of the Bavarian State Medical Association approved the study for the REGIOMED Klinikum Coburg. In the network of the University Hospital Nottingham, ASST GOM Niguarda, Warsaw and Zagreb this information was published as public statistical information.
Table 1: Basic information about included patients.
| Cohort name |
Hospital name |
Total number
of patients |
Included in the study |
Sex (f/m) |
Age (median, range) |
| Barcelona |
Hospital del Mar |
1.999 |
1.786 |
969/817 |
57 (17 – 101) |
| Bergamo |
ASST Papa Giovanni XXIII Hospital |
2.249 |
995 |
265/730 |
70 (6 – 95) |
| Coburg |
REGIOMED |
89 |
89 |
48/41 |
75 (18 – 98) |
| Helsinki |
|
100 |
100 |
44/56 |
54,5 (16 – 84) |
| Milan |
Asst GOM Niguarda |
713 |
685 |
242/443 |
63 (0 – 96) |
| Nottingham |
Nottingham University Hospitals |
795 |
795 |
356/439 |
75 (0 – 102) |
| Warsaw |
Central Clinical Hospital of Ministry of the Interior and Administration |
122 |
122 |
45/77 |
69 (19 – 96) |
| Zagreb |
Clinical Hospital Dubrava & University Hospital for Infectious Diseases |
237 |
237 |
93/144 |
63 (22 – 99) |
| Zhejiang |
|
610 |
608 |
297/311 |
49 (18 – 93) |
COVID Symptom Study app
The app COVID-Symptom Study (12) was developed by Zoe with scientific input from researchers and medical professionals at King's College London and Massachusetts General Hospital (https: // covid. Joinzoe.com/). It was launched in the UK on March 24, 2020 and reached over 3.9 million subscribers in 3 months. It enables the collection of self-reports on COVID-19 infections, as reported above (12). It is important that participants enrolled in ongoing epidemiological studies, clinical cohorts, or clinical studies provide informed consent to linking data collected through the app in accordance with HIPAA and the GDPR with existing study data that they previously provided have or could provide in the future. The code of conduct for the app was approved by the ethics committee of King's College London (REMAS ID 18210, review reference LRS-19 / 20-18210) and all users gave their consent to non-commercial use. For this work we included participants from Great Britain who started with a healthy condition and later developed symptoms that were based on the methods described in Menni et al. The presented disease score led to a suspicion of COVID-19. (12). To get an estimate of the duration of the illness, the time at which the illness ended was either the last reporting day before the end of use of the app or the first healthy day if six consecutive healthy days were reported afterwards. In order to avoid censoring, only participants with an illness duration of 30 < days and with an onset of illness before May 17 were included in the analysis (37,187 persons). Severity was calculated as the weighted average of symptoms at the time of the onset of the disease, using the normalized ratio of the frequency of symptoms at the time of the height of the disease for those who reported hospital visits after the onset of the disease and those who did not.
Data related to seasonal changes
The ambient temperature data was obtained from Climate Data Online [National Centers for Environmental Information (NCEI) database] https://www.ncdc.noaa.gov/cdo-web/.
Statistical Methods
The data collected from seven cohorts are summarized in Table 1. Patients without information on the result were excluded from the analysis. The influence of the admission date and the local ambient temperature on the change in mortality was estimated using logistic regression.
The following patient characteristics and hospital stay covariates were examined: The Died / Discharged result was used as the dependent variable and the admission as the independent variable along with age (in years) and gender (female / male). We then used the same approach for estimating the effect of ambient temperature on the need for ICU admission and for mechanical ventilation therapy. A linear model was then used to estimate the effect of ambient temperature on length of hospital stay (in days) as the dependent variable and date of admission as the independent variable along with age and gender. Before the analysis, a data transformation was performed in which the length of hospital stay was increased by 1 (due to zeros) and log10 transformed (zero days of hospital stay correspond to a hospital stay of less than 24 hours). A linear regression with mean duration as the dependent variable and the 2 week period as the independent variable was fitted to assess change over time.
For each dependent variable, the raw data were presented with bar charts (death, intensive care unit and mechanical ventilation) or box and whisker charts (length of hospital stay) for patients in 2-week groups. The filling of the bars and boxes reflects the number of patients admitted to the hospital in a specific 2-week group. Individual data points were recorded for groups with fewer than five patients.
The coefficients estimated in the logistic regression and the linear regression were combined with the help of an inverse variance-weighted meta-analysis, with models with random effects being used in view of the heterogeneity of the cohorts (R package "metaphor").
The results of the meta-analysis were presented as forest plots, which were created with the R package "ggplot2". All statistical analyzes were carried out in the programming software R (version 3.6.3), with the exception of the logistic and linear regressions for the data of the Milano cohort, which were carried out in the statistical software Stata (version 12), and the COVID cohort of the Symptom Study for which the linear regression was carried out with the Python package statsmodels (version 0.11.1).
Results
With the aim of assessing the seasonal nature of COVID-19, we evaluated the disease progression in 6,914 people from nine cohorts admitted to hospitals in Europe and China (
Tabel 1).
To avoid sampling bias, all hospitalizations that resulted in either death or medical discharge were included in the analysis. Actual numbers of patients who died and patients who recovered (grouped in 2-week intervals) since the beginning of the epidemic until the final follow-up date for reliable data capture reporting final outcome was available are presented in Figure 1A for each of the hospitals. Meta-analysis of the effect of admission date on the mortality is presented in Figure 1B. The most significant change was observed in Barcelona, where mortality odds decreased by 4.1% per day (p < 0.001). Weighted average decrease in mortality odds across all studied hospitals was 1.9% per day (p < 0.001). Our model included age as a co-variate, so this change is unlikely to be accounted for by change in age of patients. To further confirm that age was not underlying the observed changes, we analyzed age of patients admitted to hospitals in different periods and demonstrated that change in the age of patients was not a factor that could explain the observed decrease in mortality (
Supplementary Figure 1).
Since there is no standard measure or classification of COVID-19 severity used across all hospitals, to further evaluate disease severity, we analyzed secondary outcomes. We compared the duration of hospitalization, need for ICU and separately mechanical ventilation. Strong and statistically significant decrease in the duration of hospitalization was observed in Barcelona, Coburg, Milano, Nottingham, and Zagreb. In Helsinki, Warsaw, and Zhejiang, the change was in the same direction but was not statistically significant. The only outlier was Bergamo, where the change was in the opposite direction, but the change was not statistically significant (
Supplementary Figure 2). In meta-analysis, the decrease in lengths of hospitalization was statistically significant (10b = 0.995; CI = 0.991–0.998; p = 0.007). The odds to need of intensive care decreased in all hospitals in Europe and was individually statistically significant in all hospitals beside Bergamo, Helsinki, and Zagreb (
Supplementary Figure 3). Meta-analysis of European hospitals estimated that the odds to need the intensive care decreased by 2.2% per day of change in the admission date (OR = 0.978; CI = 0.962–0.993; p = 0.008) and the odds to need mechanical ventilation decreased 2.1% (OR = 0.979; CI = 0.964–0.994; p = 0.008) per day of change in the admission date (
Supplementary Figure 4).
While all hospitals in Europe were basically displaying the same trend of decreasing COVID-19 severity with time, in Zhejiang hospitals, there was either no change, or the changes trended non-significantly in opposite direction to European centers. The most notable difference between the COVID-19 pandemic in Europe and in China was that while the epidemic was entirely during winter in China, it covered both winter and spring periods in Europe. To evaluate whether weather was an important factor, we correlated the observed changes with local ambient temperature. Minimal and maximal local temperatures for all hospitals are presented in
Supplementary Figure 5. To evaluate whether the change in temperature may have been responsible for the observed changes in disease severity, we modeled mortality with ambient temperature instead of admission date.
The results presented in
Figure 2 suggest a strong effect of ambient temperature on the mortality risk (OR = 0.854 per 1°C; CI = 0.773–0.944; p = 0.007).
To further verify the change of COVID-19 with time, we analyzed individual symptom data for 37,187 participants of the COVID Symptom study app. Although there is also a sampling bias in that study, it is different from bias in hospitalization, so it was reassuring to observe a gradual decrease in duration of symptoms and COVID-19 severity in April and May (
Figure 3).
An assessment of the slope of duration as a function of time (2 ISO week) showed a significant decrease in duration (B = −0.7 p = 0.006). Regarding severity, while not overall significant (−0.0014 p = 0.836), the trend toward a decrease was stronger when considering the latest period (point 1 to the end slope = −0.0112 p = 0.116).
Discussion
By analyzing hospital records of 6,914 patients admitted to eight European hospitals, we observed a strong and statistically significant decrease in COVID-19 mortality and severity with time. Possible change in the average age of patients in different stages of the pandemic is the first obvious explanation for the decreased severity, since age is the strongest predictor of COVID-19 severity [with up to 100-fold difference in mortality risk (13)]. However, age was included in our model as a co-variate and furthermore the average age of patients did not change with time (
Supplementary Figure), so we excluded this hypothesis. An alternative explanation could be that there was change in policies for admission and/or release of COVID-19 patients during the evaluated period—possibly due to “overwhelming” medical facilities. This might have been particularly relevant in the situation of limited hospital capacity, when hospitalization may have been preceded with a triage process to identify patients who might benefit from hospitalization, admission to ICU or mechanical ventilation. However, the only hospital in our cohort that reached full capacity was Bergamo, while all others operated well below the maximal capacity for either hospitalization, or ICU, which suggests that changes in hospital admission policy were not a major driver behind the observed change in COVID-19 mortality and severity. This conclusion is further supported by concurrent decrease in duration and severity of symptoms of non-hospitalized individuals reporting symptoms in the COVID-Symptom Study Application (
Figure 3). Change in COVID-19 management, also, could have resulted in decreased severity. However, all these changes were hospital-specific, and in the analyzed period, the most effective improvement in therapy was the introduction of dexamethasone, which was reported to reduce mortality from 24.6 to 21.6% (14). As we are learning more about COVID-19, patients are receiving better and better treatment, but the progress so far was not too large, which is particularly evident from the increased mortality in the second wave in Australia [case fatality rate (CFR) was 0.5% in the first wave (15) and 3.1% in the second wave (16)]. Therefore, it is hard to imagine that minor modifications in patient management could have significantly contributed to the observed decrease in disease mortality and severity in Europe.
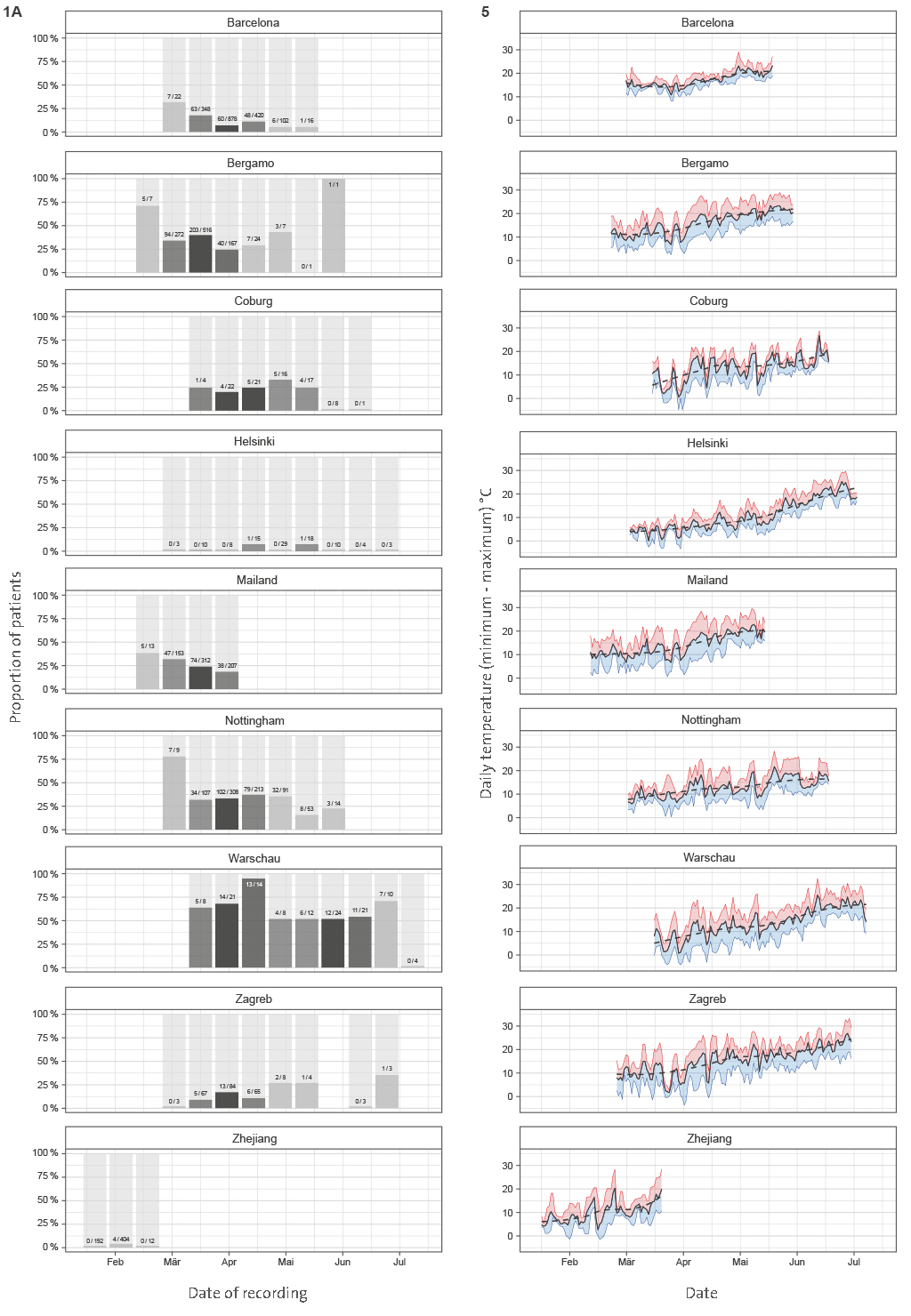
Figure 1 A | Mortality in people admitted in hospitals with COVID-19. (A) Hospitalization outcome (death/discharge) depending on the admission date (grouped in 2-week intervals) since the beginning of the pandemic.
Supplementary Figure 5 | Daily ambient temperatures in the study period. Solid black line -
Average daily temperature, red line - daily maximum, blue line - daily minimum. Dashed
black line - locally estimated scatter plot smoothing of daily average temperature. data
were published by Climate Data Online (National Centers for Environmental
Information database(NCEI): https://www.ncdc.noaa.gov/cdo-web/
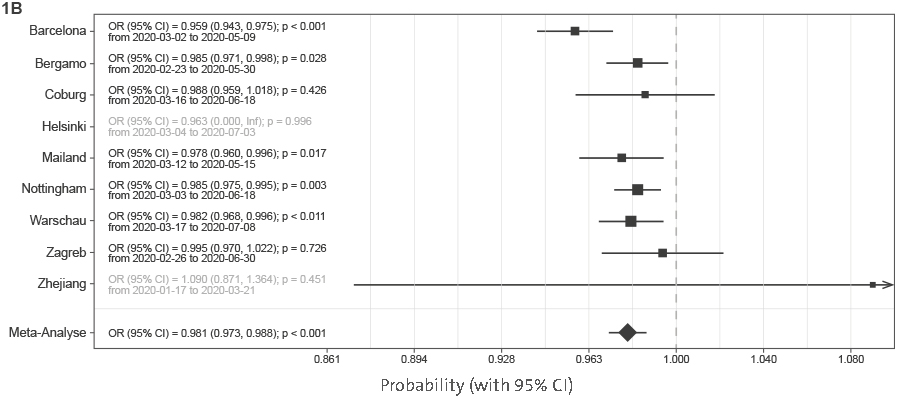
Figure 1 B | Mortality in people admitted in hospitals with COVID-19. (B) Meta-analysis of the effects of admission date on mortality (presented as odds ratios per 1-day increase in admission date). In Helsinki, there were only two deaths, and in Zhejiang hospitals, four deaths, so they were not included in the meta-analysis. OR, odds ratio; CI, confidence interval.
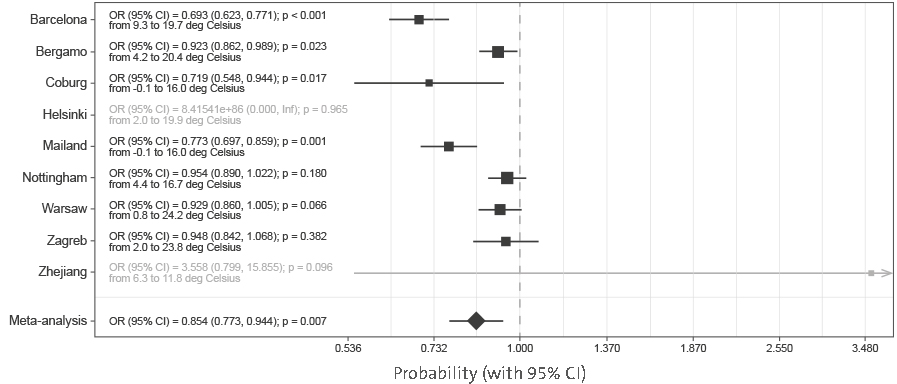
Figure 2 | Meta-analysis of the effects of temperature on mortality (presented as odds ratios per 1°C increase in average daily temperature during hospitalization). In Helsinki, there were only two deaths, and in Zhejiang hospitals, four deaths, so they were not included in the meta-analysis. OR, odds ratio; CI, confidence interval.
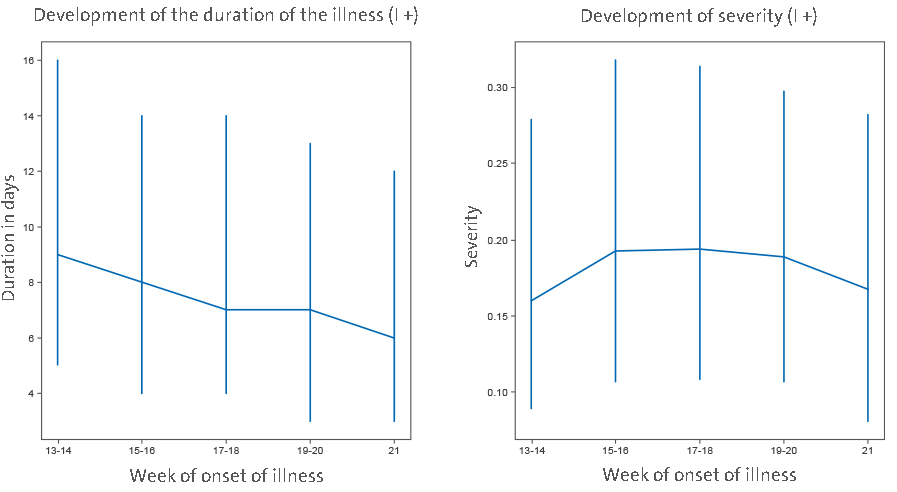
Figure 3 | Data from 37,187 individuals/suspected COVID positives (I+) recording symptoms in the COVID Symptom Study application in the United Kingdom suggest that both severity (measured as weighted sum of symptoms accounting for difference at disease peak between those reporting hospital visit and those who do not) and duration of disease symptoms slightly decrease in the United Kingdom (median values and interquartile ranges are shown). Imputed status defined as per the application of the predictive model described by Menni et al. (12) was chosen over definite PCR diagnostic in order to avoid confounding factor
of test access policy changes.
After excluding these three causes for a Europe-wide decrease in disease severity and mortality in the period from March to June, the change in season surfaced as the most probable explanation since in all studied locations ambient temperature increased considerably in that period (
Supplementary Figure 5). Exchanging hospital admission date with local temperature (
Figure 2) showed that temperature strongly correlated with decrease in COVID-19 mortality. Since reverse causation is not possible, it is reasonable to conclude that COVID-19 as a disease has a strong seasonal nature.
Despite the fact that most human coronaviruses are highly seasonal (17), the seasonal nature of COVID-19 is frequently challenged with the fact that numerous cases have been reported in tropical countries and that virus evidently can also be efficiently transmitted in hot and humid climates.
However, in all these countries, the disease mortality and severity are very low (e.g., Singapore reported 26 deaths and over 44,000 confirmed infections), which actually suggests that there may be seasonal or climate-related differences in severity of COVID-19. It is possible that the same is the case for other respiratory viruses that show strong seasonality, but asymptomatic people are generally not tested for the presence of viral RNA in the nose; thus, viral transmission, outside of their season, was not observed. The notable exception that confirms this hypothesis was the 2009 swine flu pandemic in England when numerous PCR tests were also performed in the summer. These tests revealed infection in over 250,000 people in the summer wave, but with much lower mortality than in the wither wave (18). The large increase in the number of people with positive SARS-CoV-2 PCR tests in Europe in late summer and early autumn 2020 is not accompanied by the corresponding increase in deaths. The increase in number of cases and change in the age distribution of patients (19) have been suggested as possible explanation for the missing deaths. However, in Australia, which has reverse seasons, the situation was the opposite, and the mortality was much higher in the second (winter) wave of the pandemic. Despite increased testing and global increase in the knowledge on how to treat patients, CFR in the second (winter) wave was six times higher than in the first (summer) wave [“winter” CFR was 3.1% (16) compared to “summer” CFR, which was only 0.5% (15)].
It is very difficult to prove causality in an observational study, particularly when many correlated factors are changed in the same time, but the observed decrease in COVID-19 severity with the end of winter fits very well with the known effects of outside temperature on indoor humidity and consequential restoration of mucosal barrier function, which is often impaired by dry air during the heating season (20). Most respiratory viruses peak in winter and fluctuation of temperature and humidity has been proposed as the most potent drivers of seasonality, especially in the context of the epidemic in the winter season (17). However, the peak of infection and the severity of the disease are not always fully aligned. For example, although infection rates of rhinoviruses peak in spring and fall, the disease severity increases in winter (21). Seasonal appearance of respiratory viruses is often attributed to seasonal indoor crowding and effects of temperature and humidity on stability of viral particles (22), with effect of low air humidity on the mucosal barrier often neglected.
While often considered to be a physical barrier, mucus is actually an active biological barrier that crosslinks viruses and bacteria to mucins, a group of highly glycosylated proteins that are secreted to our mucosal barriers where they self-assemble into long polymers (23). Mucin glycans mimic cell surface glycosylation and, by acting as a decoy for viral lectins, trap viral particles, which are then transported out of airways by mucociliary clearance (24). Furthermore, since all envelope viruses are highly glycosylated, a number of lectins like trefoil factors (TFF) are secreted to mucous where they crosslink viruses by binding to glycans on both viruses and mucins (25). However, this barrier is functional only if it is well hydrated to both maintain its structural integrity and enable constant flow of mucus that remove viruses and other pathogens from our airways (24).
If exposed to dry air, these barriers dry out and cannot perform their protective functions (26).
Animal experiments demonstrated the importance of humidity for both transfection of respiratory viruses and disease severity (27–29), while population-level studies in the United States indicated the importance of humidity for influenza transmission (30). One of these studies demonstrated that increasing relative humidity from 20 to 50% can significantly decrease mortality from influenza infections (29). In another study, humidification of air in obstructive sleep apnea patients reduced nasal symptoms by 60% (31), which all suggest that protective effects of humidity on mucosal barrier may be a dominant molecular mechanism behind seasonality of respiratory viruses.
A large part of human inter-individual differences are glycan-based and glycan diversity represent one of the main defenses of all higher organisms against pathogens (32). Glycans (which are covalently attached to most proteins) are chemical structures that are being inherited as complex traits, which enables diversity and significant inter-individual differences (33). SARS-CoV-2 spike glycoprotein is heavily glycosylated (34), and it was reported to bind to glycosaminoglycans (35) and sialylated glycans (36). ABO blood antigens are also glycans and are probably the best known example of glycan diversity; interestingly, people with blood type A and, thus, having one N-acetylgalactosamine more than type O are more susceptible to COVID-19 (37). All these suggest that, like most other viruses, SARS-CoV-2 is also dependent on glycans for transmission, which further support the importance of mucins and functional mucosal barrier in COVID-19.
Mucociliary dysfunction and respiratory barrier impairment promotes both initial infection and expansion of viruses within the airways of an infected individual (29). Dry air inhalation significantly decreases nasal mucociliary transition time (NMTT) in heathy individuals (38), affecting the duration of viral exposure on nasal mucosa. Nasal epithelial cells are the main portal for the initial infection and transmission of SARS-CoV-2 (39). Patients who developed clinically relevant infection after experimental transnasal viral challenge (Rhinovirus or Infl. B) had reduced epithelial barrier function (increased transepithelial resistance, reduced number of ciliated cells, and increased NMTT compared to those who were not infected or had a mild form) (40). However, experimental viral infection in vitro resulted only in decreased number of ciliated cells, without affecting tight junction protein expression (41). This controversy between in vivo and in vitro experiments suggests the importance of immune response in the control of epithelial barrier function (42). Recent studies on the interaction between climate changes and respiratory barrier dysfunction indicated not only higher incidence of viral infection but also higher vulnerability of nasal mucosa through increased incidence of nosebleed in the emergency departments in the conditions of low temperature and low humidity (43). A recently published study that exposed volunteers to respiratory syncytial virus (RSV), one of the pathogens responsible for the common cold, demonstrated that pre-existing inflammation in the respiratory mucosa was a risk factor for infection (44), which further supports the importance of mucociliary dysfunction and respiratory barrier impairment for infection with respiratory viruses.
Limitations
Potential sampling bias is the main limitation of this study. By focusing on individual progression of the disease in already hospitalized patients, we excluded effects of the unknown number of true infections on national mortality rates, and we still cannot exclude the possibility that some other unidentified external factors (including confinement and social distancing, improvement and compliance of prevention and environmental hygiene protocols, and even decreased air pollution, which could have progressively affected the severity of patients arriving to the hospital) were affecting composition of hospitalized patient cohorts and contributing to the decreased COVID-19 severity and mortality. Therefore, it is important that tracking of individual symptoms in 37,187 UK patients are showing the same trend, since these are individuals voluntarily reporting symptoms and potential sampling bias that is independent from bias in hospitalization. The choice to include imputed positives was mostly motivated by the restriction in testing access that were observed over the first wave before being relaxed in May and June. Accounting only for PCR-tested positive reporting to the app would have unduly biased the results toward higher severity in the early days. We adopted instead the model developed by Menni et al. (12) that achieved a reasonable performance in prediction of positive cases (ROC-AUC 76%).
Conclusions
Our data suggest that, in addition to affecting viral transmission, environmental factors also play an important role in already infected patients. Severity of COVID-19 decreased with the onset of spring, which paints a grim picture for the incoming winter and suggests that both disease severity and mortality may increase significantly. Since many hospitals have very dry air in winter, providing humidified air to patients in early stages of the disease may be beneficial. Considering the evident detrimental effect of dry air on our mucosal barrier and its role as the first line of defense against infection (45), in the situation of the rapidly progressing COVID-19 pandemic, it would be essential to actively promote universal humidification of dry air in all public and private heated spaces as well as active nasal hygiene and hydration (46). Humidity should also be monitored in cooled buildings with limited access to outside air, since air-conditioning is also an effective dehumidification and can result in very dry air.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.





