Original title: 2019 Novel Coronavirus (COVID-19) Pandemic: Built Environment Considerations To Reduce Transmission
Source link: https://msystems.asm.org/content/5/2/e00245-20#ref-list-1
Released: March 2020
1. Parrish CR, Holmes EC, Morens DM, Park E-C, Burke DS, Calisher CH, Laughlin CA, Saif LJ, Daszak P. 2008. Cross-species virus transmission and the emergence of new epidemic diseases. Microbiol Mol Biol Rev 72:457–470. doi:10.1128/MMBR.00004-08.Abstract/FREE Full TextGoogle Scholar
2. de Groot RJ, Baker SC, Baric RS, Brown CS, Drosten C, Enjuanes L, Fouchier RAM, Galiano M, Gorbalenya AE, Memish ZA, Perlman S, Poon LLM, Snijder EJ, Stephens GM, Woo PCY, Zaki AM, Zambon M, Ziebuhr J. 2013. Commentary: Middle East respiratory syndrome coronavirus (MERS-CoV): announcement of the Coronavirus Study Group. J Virol 87:7790–7792. doi:10.1128/JVI.01244-13.FREE Full TextGoogle Scholar
3. Peiris JSM, Lai ST, Poon LLM, Guan Y, Yam LYC, Lim W, Nicholls J, Yee WKS, Yan WW, Cheung MT, Cheng VCC, Chan KH, Tsang DNC, Yung RWH, Ng TK, Yuen KY, SARS Study Group. 2003. Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet 361:1319–1325. doi:10.1016/S0140-6736(03)13077-2.CrossRefPubMedWeb of ScienceGoogle Scholar
4. Hui DSC, Chan MCH, Wu AK, Ng PC. 2004. Severe acute respiratory syndrome (SARS): epidemiology and clinical features. Postgrad Med J 80:373–381. doi:10.1136/pgmj.2004.020263.Abstract/FREE Full TextGoogle Scholar
5. World Health Organization. 5 January 2020. Pneumonia of unknown cause – China. World Health Organization, Geneva, Switzerland.Google Scholar
6. Peeri NC, Shrestha N, Rahman MS, Zaki R, Tan Z, Bibi S, Baghbanzadeh M, Aghamohammadi N, Zhang W, Haque U. 22 February 2020. The SARS, MERS and novel coronavirus (COVID-19) epidemics, the newest and biggest global health threats: what lessons have we learned? Int J Epidemiol doi:10.1093/ije/dyaa033.CrossRefGoogle Scholar
7. Ramadan N, Shaib H. 2019. Middle East respiratory syndrome coronavirus (MERS-CoV): a review. Germs 9:35–42. doi:10.18683/germs.2019.1155.CrossRefGoogle Scholar
8. Wu P, Hao X, Lau EHY, Wong JY, Leung KSM, Wu JT, Cowling BJ, Leung GM. 2020. Real-time tentative assessment of the epidemiological characteristics of novel coronavirus infections in Wuhan, China, as at 22 January 2020. Euro Surveill 25:2000044. https://www.eurosurveillance.org/content/10.2807/1560-7917.ES.2020.25.3.2000044.CrossRefPubMedGoogle Scholar
9. Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, Ren R, Leung KSM, Lau EHY, Wong JY, Xing X, Xiang N, Wu Y, Li C, Chen Q, Li D, Liu T, Zhao J, Li M, Tu W, Chen C, Jin L, Yang R, Wang Q, Zhou S, Wang R, Liu H, Luo Y, Liu Y, Shao G, Li H, Tao Z, Yang Y, Deng Z, Liu B, Ma Z, Zhang Y, Shi G, Lam TTY, Wu JTK, Gao GF, Cowling BJ, Yang G, Leung GM, Feng Z. 29 January 2020. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. N Engl J Med doi:10.1056/NEJMoa2001316.CrossRefPubMedGoogle Scholar
10. Rothan HA, Byrareddy SN. 26 February 2020. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun doi:10.1016/j.jaut.2020.102433.CrossRefPubMedGoogle Scholar
11. Sizun J, Yu MW, Talbot PJ. 2000. Survival of human coronaviruses 229E and OC43 in suspension and after drying on surfaces: a possible source of hospital-acquired infections. J Hosp Infect 46:55–60. doi:10.1053/jhin.2000.0795.CrossRefPubMedWeb of ScienceGoogle Scholar
12. Chen Y, Liu Q, Guo D. 2020. Emerging coronaviruses: genome structure, replication, and pathogenesis. J Med Virol 92:418–423. doi:10.1002/jmv.25681.CrossRefPubMedGoogle Scholar
13. Chan J-W, Yuan S, Kok K-H, To KK-W, Chu H, Yang J, Xing F, Liu J, Yip CC-Y, Poon R-S, Tsoi H-W, Lo S-F, Chan K-H, Poon V-M, Chan W-M, Ip JD, Cai J-P, Cheng V-C, Chen H, Hui C-M, Yuen K-Y. 2020. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet 395:514–523. doi:10.1016/S0140-6736(20)30154-9.CrossRefPubMedGoogle Scholar
14. Fehr AR, Perlman S. 2015. Coronaviruses: an overview of their replication and pathogenesis. Methods Mol Biol 1282:1–23. doi:10.1007/978-1-4939-2438-7_1.CrossRefPubMedGoogle Scholar
15. Walls AC, Park Y-J, Tortorici MA, Wall A, McGuire AT, Veesler D. 2020. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell 180:1–12.Google Scholar
16. South China Agricultural University. 2020. Pangolin is found as a potential intermediate host of new coronavirus in South China Agricultural University. https://scau.edu.cn/2020/0207/c1300a219015/page.htm.Google Scholar
17. Cui J, Li F, Shi Z-L. 2019. Origin and evolution of pathogenic coronaviruses. Nat Rev Microbiol 17:181–192. doi:10.1038/s41579-018-0118-9.CrossRefPubMedGoogle Scholar
18. Perlman S. 2020. Another decade, another coronavirus. N Engl J Med 382:760–762. doi:10.1056/NEJMe2001126.CrossRefGoogle Scholar
19. Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W, China Novel Coronavirus Investigating and Research Team. 2020. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 382:727–733. doi:10.1056/NEJMoa2001017.CrossRefPubMedGoogle Scholar
20. CDC. 2020. 2019-nCoV real-time RT-PCR diagnostic panel (CDC) - fact sheet for healthcare providers. Centers for Disease Control and Prevention, Atlanta, GA.Google Scholar
21. Millán-Oñate J, Rodriguez-Morales AJ, Camacho-Moreno G, Mendoza-Ramírez H, Rodríguez-Sabogal IA, Álvarez-Moreno C. A new emerging zoonotic virus of concern: the 2019 novel coronavirus (COVID-19). Infectio, in press.Google Scholar
22. Horve PF, Lloyd S, Mhuireach GA, Dietz L, Fretz M, MacCrone G, Van Den Wymelenberg K, Ishaq SL. 2020. Building upon current knowledge and techniques of indoor microbiology to construct the next era of theory into microorganisms, health, and the built environment. J Expo Sci Environ Epidemiol 30:219–217. doi:10.1038/s41370-019-0157-y.CrossRefGoogle Scholar
23. Adams RI, Bhangar S, Dannemiller KC, Eisen JA, Fierer N, Gilbert JA, Green JL, Marr LC, Miller SL, Siegel JA, Stephens B, Waring MS, Bibby K. 2016. Ten questions concerning the microbiomes of buildings. Build Environ 109:224–234. doi:10.1016/j.buildenv.2016.09.001.CrossRefGoogle Scholar
24. Tellier R, Li Y, Cowling BJ, Tang JW. 2019. Recognition of aerosol transmission of infectious agents: a commentary. BMC Infect Dis 19:101. doi:10.1186/s12879-019-3707-y.CrossRefPubMedGoogle Scholar
25. Andrews JR, Morrow C, Walensky RP, Wood R. 2014. Integrating social contact and environmental data in evaluating tuberculosis transmission in a South African township. J Infect Dis 210:597–603. doi:10.1093/infdis/jiu138.CrossRefPubMedGoogle Scholar
26. Mizumoto K, Chowell G. 2020. Transmission potential of the novel coronavirus (COVID-19) onboard the Diamond Princess Cruises Ship, 2020. Infect Dis Model 5:264–270. doi:10.1016/j.idm.2020.02.003.CrossRefPubMedGoogle Scholar
27. Wu JT, Leung K, Leung GM. 2020. Nowcasting and forecasting the potential domestic and international spread of the 2019-nCoV outbreak originating in Wuhan, China: a modelling study. Lancet 395:689–697. doi:10.1016/S0140-6736(20)30260-9.CrossRefPubMedGoogle Scholar
28. Zhang S, Diao M, Yu W, Pei L, Lin Z, Chen D. 2020. Estimation of the reproductive number of novel coronavirus (COVID-19) and the probable outbreak size on the Diamond Princess cruise ship: a data-driven analysis. Int J Infect Dis 93:201–204. doi:10.1016/j.ijid.2020.02.033.CrossRefPubMedGoogle Scholar
29. Poon LLM, Peiris M. 2020. Emergence of a novel human coronavirus threatening human health. Nat Med 26:317–319. doi:10.1038/s41591-020-0796-5.CrossRefGoogle Scholar
30. Guerra FM, Bolotin S, Lim G, Heffernan J, Deeks SL, Li Y, Crowcroft NS. 2017. The basic reproduction number (R0) of measles: a systematic review. Lancet Infect Dis 17:e420–e428. doi:10.1016/S1473-3099(17)30307-9.CrossRefGoogle Scholar
31. Biggerstaff M, Cauchemez S, Reed C, Gambhir M, Finelli L. 2014. Estimates of the reproduction number for seasonal, pandemic, and zoonotic influenza: a systematic review of the literature. BMC Infect Dis 14:480. doi:10.1186/1471-2334-14-480.CrossRefPubMedGoogle Scholar
32. Zhao S, Cao P, Gao D, Zhuang Z, Chong MKC, Cai Y, Ran J, Wang K, Yang L, He D, Wang MH. 20 February 2020. Epidemic growth and reproduction number for the novel coronavirus disease (COVID-19) outbreak on the Diamond Princess Cruise Ship from January 20 to February 19, 2020: a preliminary data-driven analysis. SSRN doi:10.2139/ssrn.3543150.CrossRefGoogle Scholar
33. Mizumoto K, Kagaya K, Zarebski A, Chowell G. 2020. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-19) cases on board the Diamond Princess cruise ship, Yokohama, Japan, 2020. Euro Surveill 25(10):pii=2000180. https://www.eurosurveillance.org/content/10.2807/1560-7917.ES.2020.25.10.2000180.Google Scholar
34. Ong SWX, Tan YK, Chia PY, Lee TH, Ng OT, Wong MSY, Marimuthu K. 2020. Air, surface environmental, and personal protective equipment contamination by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from a symptomatic patient. JAMA doi:10.1001/jama.2020.3227.CrossRefGoogle Scholar
35. Stephens B, Azimi P, Thoemmes MS, Heidarinejad M, Allen JG, Gilbert JA. 2019. Microbial exchange via fomites and implications for human health. Curr Pollution Rep 5:214. doi:10.1007/s40726-019-00126-3.CrossRefGoogle Scholar
36. Vandegrift R, Fahimipour AK, Muscarella M, Bateman AC, Van Den Wymelenberg K, Bohannan B. 26 March 2019. Moving microbes: the dynamics of transient microbial residence on human skin. bioRxiv doi:10.1101/586008.Abstract/FREE Full TextGoogle Scholar
37. Rothe C, Schunk M, Sothmann P, Bretzel G, Froeschl G, Wallrauch C, Zimmer T, Thiel V, Janke C, Guggemos W, Seilmaier M, Drosten C, Vollmar P, Zwirglmaier K, Zange S, Wölfel R, Hoelscher M. 2020. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. N Engl J Med 382:970–971. doi:10.1056/NEJMc2001468.CrossRefPubMedGoogle Scholar
38. Yaqian M, Lin W, Wen J, Chen G. 2020. Epidemiological and clinical characteristics of SARS-CoV-2 and SARS-CoV: a system review. Infectious Diseases (except HIV/AIDS). medRxiv doi:10.1101/2020.02.20.20025601.Abstract/FREE Full TextGoogle Scholar
39. CDC. 2020. Coronavirus disease 2019 (COVID-19). Centers for Disease Control and Prevention, Atlanta, GA.Google Scholar
40. Doultree JC, Druce JD, Birch CJ, Bowden DS, Marshall JA. 1999. Inactivation of feline calicivirus, a Norwalk virus surrogate. J Hosp Infect 41:51–57. doi:10.1016/S0195-6701(99)90037-3.CrossRefPubMedWeb of ScienceGoogle Scholar
41. Bin SY, Heo JY, Song M-S, Lee J, Kim E-H, Park S-J, Kwon H-I, Kim SM, Kim Y-I, Si Y-J, Lee I-W, Baek YH, Choi W-S, Min J, Jeong HW, Choi YK. 2016. Environmental contamination and viral shedding in MERS patients during MERS-CoV outbreak in South Korea. Clin Infect Dis 62:755–760. doi:10.1093/cid/civ1020.CrossRefPubMedGoogle Scholar
42. Kampf G, Todt D, Pfaender S, Steinmann E. 2020. Persistence of coronaviruses on inanimate surfaces and its inactivation with biocidal agents. J Hosp Infect 104:246–251. doi:10.1016/j.jhin.2020.01.022.CrossRefPubMedGoogle Scholar
43. van Doremalen N, Bushmaker T, Morris DH, Holbrook MG, Gamble A, Williamson BN, Tamin A, Harcourt JL, Thornburg NJ, Gerber SI, Lloyd-Smith JO, de Wit E, Munster VJ. 2020. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med doi:10.1056/NEJMc2004973.CrossRefPubMedGoogle Scholar
44. Xiao F, Tang M, Zheng X, Liu Y, Li X, Shan H. 2020. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology doi:10.1053/j.gastro.2020.02.055.CrossRefPubMedGoogle Scholar
45. Lipsitch M, Allen J. 16 March 2020. Coronavirus reality check: 7 myths about social distancing, busted. USA Today, McLean, VA. https://www.usatoday.com/story/opinion/2020/03/16/coronavirus-social-distancing-myths-realities-column/5053696002/.Google Scholar
46. Bell DM, World Health Organization Working Group on International and Community Transmission of SARS. 2004. Public health interventions and SARS spread, 2003. Emerg Infect Dis 10:1900–1906. doi:10.3201/eid1011.040729.CrossRefPubMedWeb of ScienceGoogle Scholar
47. Coronaviridae Study Group of the International Committee on Taxonomy of Viruses. 2020. The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol doi:10.1038/s41564-020-0695-z.CrossRefPubMedGoogle Scholar
48. Chang D, Xu H, Rebaza A, Sharma L, Dela Cruz CS. 2020. Protecting health-care workers from subclinical coronavirus infection. Lancet Respir Med 8:e13. doi:10.1016/S2213-2600(20)30066-7.CrossRefGoogle Scholar
49. Booth TF, Kournikakis B, Bastien N, Ho J, Kobasa D, Stadnyk L, Li Y, Spence M, Paton S, Henry B, Mederski B, White D, Low DE, McGeer A, Simor A, Vearncombe M, Downey J, Jamieson FB, Tang P, Plummer F. 2005. Detection of airborne severe acute respiratory syndrome (SARS) coronavirus and environmental contamination in SARS outbreak units. J Infect Dis 191:1472–1477. doi:10.1086/429634.CrossRefPubMedWeb of ScienceGoogle Scholar
50. American Society of Heating, Refrigerating and Air Condition Engineers, Inc. (ASHRAE). 2017. Ventilation of health care facilities (ANSI/ASHRAE/ASHE standard 170-2017). American Society of Heating, Refrigerating and Air Condition Engineers, Inc., Atlanta, GA.Google Scholar
51. Institute of Environmental Sciences and Technology. 2016. HEPA and ULPA Filters (IEST-RP-CC001.6). Institute of Environmental Sciences and Technology, Schaumburg, IL.Google Scholar
52. Goldsmith CS, Tatti KM, Ksiazek TG, Rollin PE, Comer JA, Lee WW, Rota PA, Bankamp B, Bellini WJ, Zaki SR. 2004. Ultrastructural characterization of SARS coronavirus. Emerg Infect Dis 10:320–326. doi:10.3201/eid1002.030913.CrossRefPubMedWeb of ScienceGoogle Scholar
53. Knowles H. 3 July 2019. Mold infections leave one dead and force closure of operating rooms at children’s hospital. Washington Post, Washington, DC.Google Scholar
54. So RCH, Ko J, Yuan YWY, Lam JJ, Louie L. 2004. Severe acute respiratory syndrome and sport: facts and fallacies. Sports Med 34:1023–1033. doi:10.2165/00007256-200434150-00002.CrossRefPubMedGoogle Scholar
55. Goldberg JL. 2017. Guideline implementation: hand hygiene. AORN J 105:203–212. doi:10.1016/j.aorn.2016.12.010.CrossRefGoogle Scholar
56. Chaovavanich A, Wongsawat J, Dowell SF, Inthong Y, Sangsajja C, Sanguanwongse N, Martin MT, Limpakarnjanarat K, Sirirat L, Waicharoen S, Chittaganpitch M, Thawatsupha P, Auwanit W, Sawanpanyalert P, Melgaard B. 2004. Early containment of severe acute respiratory syndrome (SARS); experience from Bamrasnaradura Institute, Thailand. J Med Assoc Thai 87:1182–1187.PubMedGoogle Scholar
57. Center for Devices, Radiological Health. 2020. N95 respirators and surgical masks (face masks). US Food and Drug Administration, Silver Spring, MD.Google Scholar
58. Centers for Disease Control and Prevention. 2020. Interim guidance for the use of masks to control seasonal influenza virus transmission. Centers for Disease Control and Prevention, Atlanta, GA.Google Scholar
59. Ryu S, Gao H, Wong JY, Shiu EYC, Xiao J, Fong MW, Cowling BJ. 2020. Nonpharmaceutical measures for pandemic influenza in nonhealthcare settings-international travel-related measures. Emerg Infect Dis doi:10.3201/eid2605.190993.CrossRefGoogle Scholar
60. Fong MW, Gao H, Wong JY, Xiao J, Shiu EYC, Ryu S, Cowling BJ. 2020. Nonpharmaceutical measures for pandemic influenza in nonhealthcare settings-social distancing measures. Emerg Infect Dis doi:10.3201/eid2605.190995.CrossRefGoogle Scholar
61. Vandegrift R, Bateman AC, Siemens KN, Nguyen M, Wilson HE, Green JL, Van Den Wymelenberg KG, Hickey RJ. 2017. Cleanliness in context: reconciling hygiene with a modern microbial perspective. Microbiome 5:76. doi:10.1186/s40168-017-0294-2.CrossRefGoogle Scholar
62. Qian H, Zheng X. 2018. Ventilation control for airborne transmission of human exhaled bio-aerosols in buildings. J Thorac Dis 10:S2295–S2304. doi:10.21037/jtd.2018.01.24.CrossRefGoogle Scholar
63. Kim SW, Ramakrishnan MA, Raynor PC, Goyal SM. 2007. Effects of humidity and other factors on the generation and sampling of a coronavirus aerosol. Aerobiologia 23:239–248. doi:10.1007/s10453-007-9068-9.CrossRefGoogle Scholar
64. Casanova LM, Jeon S, Rutala WA, Weber DJ, Sobsey MD. 2010. Effects of air temperature and relative humidity on coronavirus survival on surfaces. Appl Environ Microbiol 76:2712–2717. doi:10.1128/AEM.02291-09.Abstract/FREE Full TextGoogle Scholar
65. Chan KH, Malik Peiris JS, Lam SY, Poon LLM, Yuen KY, Seto WH. 2011. The effects of temperature and relative humidity on the viability of the SARS coronavirus. Adv Virol 2011:734690. doi:10.1155/2011/734690.CrossRefPubMedGoogle Scholar
66. BioSpace. 11 February 2020. Condair study shows indoor humidification can reduce the transmission and risk of infection from coronavirus. BioSpace, Urbandale, IA.Google Scholar
67. Noti JD, Blachere FM, McMillen CM, Lindsley WG, Kashon ML, Slaughter DR, Beezhold DH. 2013. High humidity leads to loss of infectious influenza virus from simulated coughs. PLoS One 8:e57485. doi:10.1371/journal.pone.0057485.CrossRefGoogle Scholar
68. Marr LC, Tang JW, Van Mullekom J, Lakdawala SS. 2019. Mechanistic insights into the effect of humidity on airborne influenza virus survival, transmission and incidence. J R Soc Interface 16:20180298. doi:10.1098/rsif.2018.0298.CrossRefGoogle Scholar
69. Xie X, Li Y, Chwang ATY, Ho PL, Seto WH. 2007. How far droplets can move in indoor environments–revisiting the Wells evaporation-falling curve. Indoor Air 17:211–225. doi:10.1111/j.1600-0668.2007.00469.x.CrossRefPubMedWeb of ScienceGoogle Scholar
70. Yang W, Marr LC. 2012. Mechanisms by which ambient humidity may affect viruses in aerosols. Appl Environ Microbiol 78:6781–6788. doi:10.1128/AEM.01658-12.Abstract/FREE Full TextGoogle Scholar
71. Memarzadeh F, Olmsted RN, Bartley JM. 2010. Applications of ultraviolet germicidal irradiation disinfection in health care facilities: effective adjunct, but not stand-alone technology. Am J Infect Control 38:S13–S24. doi:10.1016/j.ajic.2010.04.208.CrossRefPubMedGoogle Scholar
72. Kudo E, Song E, Yockey LJ, Rakib T, Wong PW, Homer RJ, Iwasaki A. 2019. Low ambient humidity impairs barrier function and innate resistance against influenza infection. Proc Natl Acad Sci U S A 116:10905–10910. doi:10.1073/pnas.1902840116.Abstract/FREE Full TextGoogle Scholar
73. Eccles R. 2002. An explanation for the seasonality of acute upper respiratory tract viral infections. Acta Otolaryngol 122:183–191. doi:10.1080/00016480252814207.CrossRefPubMedGoogle Scholar
74. Salah B, Dinh Xuan AT, Fouilladieu JL, Lockhart A, Regnard J. 1988. Nasal mucociliary transport in healthy subjects is slower when breathing dry air. Eur Respir J 1:852–855.Abstract/FREE Full TextGoogle Scholar
75. Block SS. 1953. Humidity requirements for mold growth. Appl Microbiol 1:287–293. doi:10.1128/AEM.1.6.287-293.1953.CrossRefPubMedGoogle Scholar
76. Kembel SW, Jones E, Kline J, Northcutt D, Stenson J, Womack AM, Bohannan BJ, Brown GZ, Green JL. 2012. Architectural design influences the diversity and structure of the built environment microbiome. ISME J 6:1469–1479. doi:10.1038/ismej.2011.211.CrossRefPubMedWeb of ScienceGoogle Scholar
77. Mhuireach GÁ, Brown GZ, Kline J, Manandhar D, Moriyama M, Northcutt D, Rivera I, Van Den Wymelenberg K. 2020. Lessons learned from implementing night ventilation of mass in a next-generation smart building. Energy Build 207:109547. doi:10.1016/j.enbuild.2019.109547.CrossRefGoogle Scholar
78. Meadow JF, Altrichter AE, Kembel SW, Kline J, Mhuireach G, Moriyama M, Northcutt D, O’Connor TK, Womack AM, Brown GZ, Green JL, Bohannan BJM. 2014. Indoor airborne bacterial communities are influenced by ventilation, occupancy, and outdoor air source. Indoor Air 24:41–48. doi:10.1111/ina.12047.CrossRefPubMedGoogle Scholar
79. Howard-Reed C, Wallace LA, Ott WR. 2002. The effect of opening windows on air change rates in two homes. J Air Waste Manag Assoc 52:147–159. doi:10.1080/10473289.2002.10470775.CrossRefPubMedGoogle Scholar
80. Fahimipour AK, Hartmann EM, Siemens A, Kline J, Levin DA, Wilson H, Betancourt-Román CM, Brown GZ, Fretz M, Northcutt D, Siemens KN, Huttenhower C, Green JL, Van Den Wymelenberg K. 2018. Daylight exposure modulates bacterial communities associated with household dust. Microbiome 6:175. doi:10.1186/s40168-018-0559-4.CrossRefGoogle Scholar
81. Schuit M, Gardner S, Wood S, Bower K, Williams G, Freeburger D, Dabisch P. 2020. The influence of simulated sunlight on the inactivation of influenza virus in aerosols. J Infect Dis 221:372–378. doi:10.1093/infdis/jiz582.CrossRefGoogle Scholar
82. Dijk D-J, Duffy JF, Silva EJ, Shanahan TL, Boivin DB, Czeisler CA. 2012. Amplitude reduction and phase shifts of melatonin, cortisol and other circadian rhythms after a gradual advance of sleep and light exposure in humans. PLoS One 7:e30037. doi:10.1371/journal.pone.0030037.CrossRefPubMedGoogle Scholar
83. Issa MH, Rankin JH, Attalla M, Christian AJ. 2011. Absenteeism, performance and occupant satisfaction with the indoor environment of green Toronto schools. Indoor Built Environ 20:511–523. doi:10.1177/1420326X11409114.CrossRefWeb of ScienceGoogle Scholar
84. Rutala WA, Weber DJ, Healthcare Infection Control Practices Advisory Committee (HIPAC). 2017. Guideline for disinfection and sterilization in healthcare facilities, 2017. Centers for Disease Control and Prevention, Atlanta, GA.Google Scholar
85. Tseng C-C, Li C-S. 2007. Inactivation of viruses on surfaces by ultraviolet germicidal irradiation. J Occup Environ Hyg 4:400–405. doi:10.1080/15459620701329012.CrossRefPubMedGoogle Scholar
86. Lytle CD, Sagripanti J-L. 2005. Predicted inactivation of viruses of relevance to biodefense by solar radiation. J Virol 79:14244–14252. doi:10.1128/JVI.79.22.14244-14252.2005.Abstract/FREE Full TextGoogle Scholar
87. Bedell K, Buchaklian AH, Perlman S. 2016. Efficacy of an automated multiple emitter whole-room ultraviolet-C disinfection system against coronaviruses MHV and MERS-CoV. Infect Control Hosp Epidemiol 37:598–599. doi:10.1017/ice.2015.348.CrossRefGoogle Scholar
88. Nardell EA, Bucher SJ, Brickner PW, Wang C, Vincent RL, Becan-McBride K, James MA, Michael M, Wright JD. 2008. Safety of upper-room ultraviolet germicidal air disinfection for room occupants: results from the Tuberculosis Ultraviolet Shelter Study. Public Health Rep 123:52–60. doi:10.1177/003335490812300108.CrossRefPubMedGoogle Scholar
89. Miller SL, Linnes J, Luongo J. 2013. Ultraviolet germicidal irradiation: future directions for air disinfection and building applications. Photochem Photobiol 89:777–781. doi:10.1111/php.12080.CrossRefGoogle Scholar
90. Welch D, Buonanno M, Grilj V, Shuryak I, Crickmore C, Bigelow AW, Randers-Pehrson G, Johnson GW, Brenner DJ. 2018. Far-UVC light: a new tool to control the spread of airborne-mediated microbial diseases. Sci Rep 8:2752. doi:10.1038/s41598-018-21058-w.CrossRefGoogle Scholar
91. Buonanno M, Stanislauskas M, Ponnaiya B, Bigelow AW, Randers-Pehrson G, Xu Y, Shuryak I, Smilenov L, Owens DM, Brenner DJ. 2016. 207-nm UV light-a promising tool for safe low-cost reduction of surgical site infections. II: In-vivo safety studies. PLoS One 11:e0138418. doi:10.1371/journal.pone.0138418.CrossRefGoogle Scholar
92. Kembel SW, Meadow JF, O’Connor TK, Mhuireach G, Northcutt D, Kline J, Moriyama M, Brown GZ, Bohannan BJM, Green JL. 2014. Architectural design drives the biogeography of indoor bacterial communities. PLoS One 9:e87093. doi:10.1371/journal.pone.0087093.CrossRefPubMedGoogle Scholar
93. NIAID. 2020. Novel coronavirus SARS-CoV-2. Flickr.Google Scholar
94. Yu IT, Li Y, Wong TW, Tam W, Chan AT, Lee JH, Leung DY, Ho T. 2004. Evidence of airborne transmission of the severe acute respiratory syndrome virus. N Engl J Med 350:1731–1739. doi:10.1056/NEJMoa032867.CrossRefPubMedWeb of ScienceGoogle Scholar
95. Li Y, Duan S, Yu ITS, Wong TW. 2004. Multi-zone modeling of probable SARS virus transmission by airflow between flats in Block E, Amoy Gardens. Indoor Air 15:96–111. doi:10.1111/j.1600-0668.2004.00318.x.CrossRefGoogle Scholar
96. Liu Y, Ning Z, Chen Y, Guo M, Liu Y, Gali NK, Sun L, Duan Y, Cai J, Westerdahl D, Liu X, Ho K-F, Kan H, Fu Q, Lan K. 2020. Aerodynamic characteristics and RNA concentration of SARS-CoV-2 aerosol in Wuhan hospitals during COVID-19 outbreak. bioRxiv doi:10.1101/2020.03.08.982637.Google Scholar

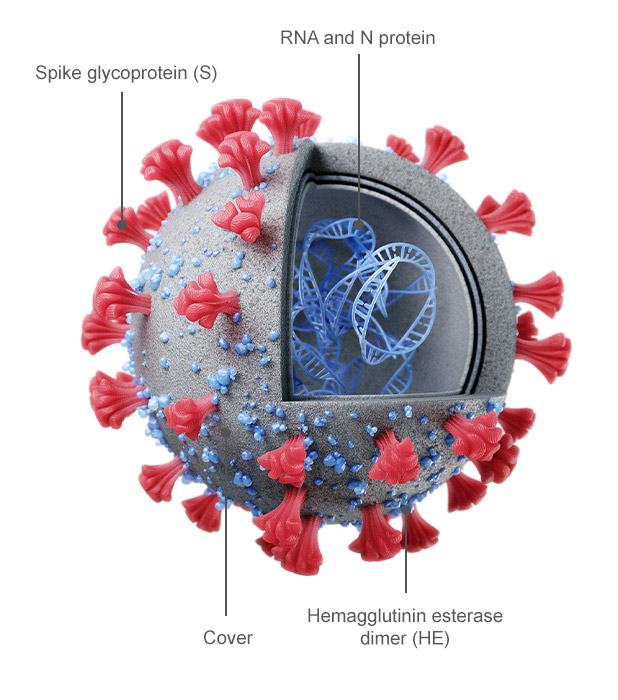 Figure 1
Figure 1 Figure 2
Figure 2 Figure 3
Figure 3