Introduction
One of the earliest accounts of the winter epidemic of respiratory infectious disease can be found in the “Book of Epidemics,” an ancient Greek record written by Hippocrates around 400 BC (1). Since then, many respiratory viruses have been identified as the etiological agents of such epidemics. Remarkable advances in virology and immunology have elucidated the underlying cause of such seasonal infections. Despite major efforts in public health, epidemics of viral respiratory tract infections continue to be highly prevalent among healthy human populations and can lead to lethal consequences in susceptible individuals. Estimated costs in the United States for the common cold are $40 billion per year (2) and over $87 billion per year for influenza (3). Furthermore, emerging virus epidemics, such as the 2002–2003 severe acute respiratory syndrome coronavirus (SARS-CoV) and the recently emerged SARS-CoV-2, occur during the winter months (4–7), indicating that the winter environment promotes the spread of a variety of respiratory virus infections.
Accumulating studies point to possible seasonal determinants in the epidemics of respiratory viruses as well as host factors affected by these contributing factors. These include seasonal changes in temperature, absolute humidity (AH), sunlight, vitamin status, and host behavior (8–16). These proposed factors can be classified as seasonal changes of environment, human behavioral patterns, and viral factors (Figure 1). Environmental factors affect host susceptibility by modulating airway defense mechanisms and affect viability and transmission of respiratory viruses. Human behavioral patterns affect the contact rates between infected individuals and susceptible individuals. Among potential drivers of seasonality, fluctuation of temperature and AH throughout the year has been proposed as a critical factor in the seasonal increase in respiratory virus infections, especially in the context of the epidemics in the winter season (12, 15–18). This review focuses on how seasonal environmental outdoor and indoor factors influence transmission and host airway response to viruses and how such changes in the host defense ultimately result in the seasonal circulation of the respiratory viruses.
Effect of outdoor seasonal climate on indoor climate
The term seasonal infection associates a specific infection with a distinct season of the year. Consequently, the perceived relationship between infections and seasonal climate is considered to be causal. This was accurate to some extent when humans lived and worked outdoors with minimal protection from even the most severe climate conditions. The industrial revolutions changed all this. Outdoor agricultural workplaces were relocated into factories and offices, moving human lifestyle away from nature and outdoor climate. With the widespread introduction of central heating and increasingly airtight, insulated building shells, a consistent thermal comfort zone could be maintained indoors, causing even further disconnection from daily and seasonal outdoor climate fluctuations. This disconnection is particularly evident in winter, when indoor heating causes a major divergence of indoor and outdoor temperature and relative humidity (RH) but does not affect AH. Measurements of indoor humidities in 40 residential apartments in New York (19) and in 6 high-quality commercial buildings in the Midwest (20) showed indoor vapor pressure of below 10 mb or indoor RH of below 24% in the winter. Thus, wintertime low AH outdoors translates into low indoor RH, within the comfort temperature range of 20 to 24°C.
The number of people-to-people contacts significantly increases on workdays compared to weekends, while local weather conditions such as rain, sunshine, and coldness have minor effects on the contacts (21). These results contradict the frequently voiced idea that indoor gathering because of nasty weather conditions has a relevant effect on the seasonality of infections. In the industrialized world, most people interact, work, sleep, commute, and spend 90% of their lifetime in enclosed spaces, where they share a limited amount of breathing air (22, 23).
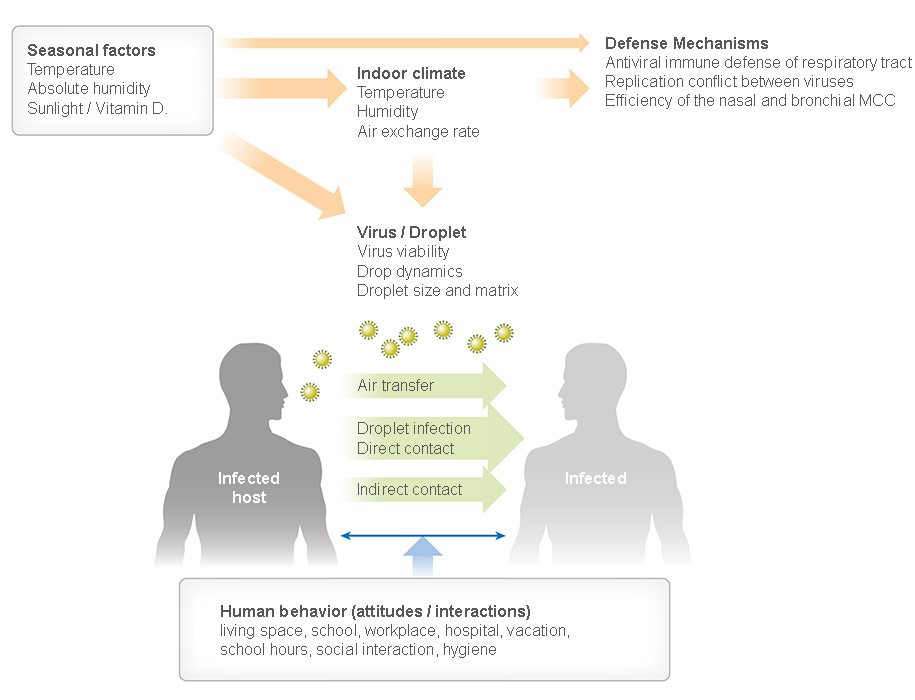
Figure 1
Factors that affect respiratory virus transmission. Seasonal environmental factors modulate host airway immune responses and affect viability and transmission ways of respiratory viruses. Human behavior affects the contact rates between infected and susceptible individuals. Abbreviations: AH, absolute humidity; MCC, mucociliary clearance; RH, relative humidity. Figure adapted from image created with BioRender.com.
This implies that the overwhelming majority of person-to-person transmission events happen indoors. The corollary implication is that indoor climate and air change rates, modulated by outdoor seasonal conditions, are the key drivers of seasonal patterns in epidemiology. In addition, exposure to outdoor conditions (albeit 10% of lifetime) contributes to alteration of respiratory defense on the existing virome (24). The multiple factors described in Figure 1 modulate the spatiotemporal onset and progression of seasonal respiratory viral infections. With this in mind and focusing on temperate regions, we discuss the importance of environmental factors on the transmission of respiratory viruses and the host immune response.
Seasonality of respiratory viruses in the human population
To date, at least nine distinct viruses have been identified as common causative agents for respiratory tract infection (25, 26). According to the epidemiological studies in temperate regions, most of the respiratory viruses have seasonal oscillation of their outbreaks (Figure 2).
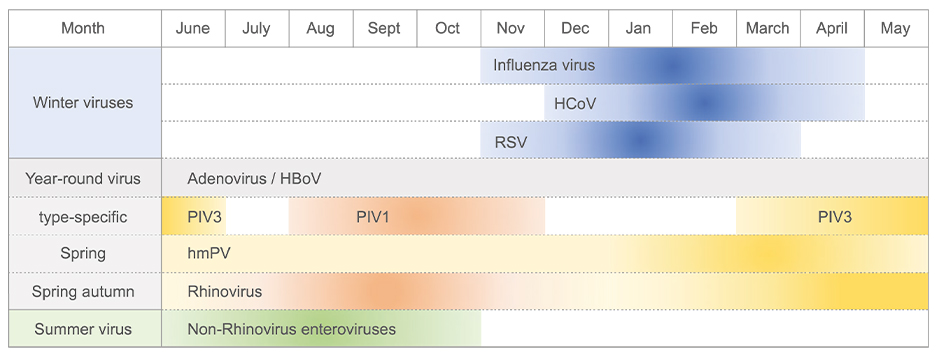
Figure 2
Schematic of seasonality of respiratory virus infection in temperate regions. Respiratory viruses are classified in three groups according to their seasonal epidemics. Influenza virus, human coronavirus (HCoV) (such as strains OC43, HKU1, 229E, and NL63), and human respiratory syncytial virus (RSV) show peaks in winter (winter viruses). Adenovirus, human bocavirus (HBoV), parainfluenza virus (PIV), human metapneumovirus (hMPV), and rhinovirus can be detected throughout the year (all-year viruses). Seasonal patterns of PIV are type specific. Epidemics of PIV type 1 (PIV1) and PIV type 3 (PIV3) peak in the fall and spring-summer, respectively. The prevalence of some non-rhinovirus enteroviruses increases in summer (summer viruses). The months indicated at the top are based on Northern Hemisphere. Figure adapted from image created with BioRender.com.
Influenza virus, human coronavirus, and human respiratory syncytial virus (RSV) clearly show peak incidences in the winter months (leading to them sometimes being called winter viruses) (14, 27–31). Conversely, adenovirus, human bocavirus, human metapneumovirus (hMPV), and rhinovirus can be detected throughout the year (all-year viruses) (30–32). For some enteroviruses, detection frequency and case numbers increase in summer (summer viruses) (33, 34). Although infection rates peak in spring and fall, disease severity caused by rhinovirus infection increases in winter (35, 36). Furthermore, parainfluenza virus (PIV) shows a type-specific pattern of seasonal circulation (37) (Figure 2).
Replication conflicts among those respiratory viruses can contribute to the nonoverlapping peak incidence with respect to one another. Interference between respiratory viruses has been recognized by epidemiological observation that influenza viruses and RSV do not share peaks during the same period even though both are prevalent in winter (38). During the influenza pandemic in 2009, rhinovirus prevalence was considered to delay the introduction of the influenza pandemic into Europe (39, 40). Using statistical approaches, a recent study shows a strong negative interaction between seasonal influenza A virus and rhinovirus at both the population and individual levels (41). Several possible mechanisms of the interference have been proposed, including disruption of cell surface viral receptor, cell death, or the host interferon (IFN) responses (41–43). Protective antibody-driven interferences have also been proposed for the conflict of genetically close viruses such as PIV, hMPV, and RSV (44).
Effect of environmental factors on stability and transmission of respiratory viruses
Respiratory virus infection can occur through (a) direct/indirect contact, (b) droplet spray in short-range transmission, or (c) aerosol in long-range transmission (airborne transmission) (45). Airborne transmission occurs as droplet spray of predominantly large droplets up to millimeters settling directly or by indirect contact on mucous membranes or by inhalation of either large respiratory droplets (>10 μm in diameter) or small airborne droplet nuclei (%lt;5 μm in diameter). The relative importance of these modes for influenza virus transmission has been reviewed (46, 47). The viral transmission efficiency through all routes is affected by indoor and outdoor seasonal environmental factors (Figure 1). In this section, we focus on the effects of environmental factors on the properties of the viral particle within the droplet matrix, especially on the stability and transmissibility of respiratory viruses.
Climate
season |
Absolute humidity (AH)
Outdoor (g/m3) |
Relative humidity (RH) Indoor (%) |
Respiratory virus stability |
Proportion of droplet nuclei |
Viability of respiratory viruses |
Predominant transmission |
| Tropical |
High |
60–100 |
High |
Low |
High |
Fomite, direct and indirect contact |
| Temperate: spring, fall |
Intermediate |
40–60 |
Low |
Low |
Low |
All transmission ways possible |
| Temperate: winter |
Low |
10–40 |
High |
High |
High |
Predominantly airborne |
TABEL 1
Droplet transfer at different relative humidity
Stability of Respiratory Viruses
There are numerous findings in current literature that correlate the viability of influenza virus, suspended within the droplet matrix, with the degree of droplet evaporation and the associated supersaturation of the enclosed ingredients (48–51). The state of vapor equilibrium in room air, expressed as saturation ratio or RH, affects all infectious droplets with respiratory viruses, independent of their source (respiratory tract or aerosolized from any fluid) and location (in air or settled on surfaces). RH therefore affects all transmission ways but has the most pronounced effect on airborne transmission. Animal transmission studies with guinea pigs and ferrets have revealed that the equilibrium state in high RH (>60%) and low RH (<40%) seems to allow viability of influenza viruses in droplets, while in intermediate RH (40% to 60%) viruses become inactivated (47, 49, 52–54) (Table 1).
It is assumed that temperature and humidity modulate the viability of viruses by affecting the properties of viral surface proteins and lipid membrane (12, 55). Viability experiments with various aerosolized respiratory viruses have been performed in aerosol chambers with controlled temperature and RH (56–61) (Supplemental Table 1). Known quantities of viruses were nebulized from solutes containing salts and proteins, and viral decay rates were measured by viral plaque assays. The results indicate a striking correlation of the stability of winter viruses at low RH (20–50%), while the stability of summer or all-year viruses is enhanced at higher RH (80%) (Supplemental Table 1). Earlier studies examined the aerosolized influenza virus viability under various temperatures and/or RH (57, 62). These studies found that temperatures in the thermal comfort zone and low RH condition, typical indoor winter features in temperate climates, slow inactivation of influenza virus. More recently, an analytical chemistry approach revealed that the low-temperature condition promotes the ordering of lipids on the viral membrane and contributes to the stability of the influenza virus particle (63).
Transmission of Respiratory Viruses
Influenza virus transmission models have been established in mice, ferrets, and guinea pigs (54, 64–68). The early studies using specialized apparatus for viral transmission between mice demonstrated the possibility that dry and unventilated air can increase opportunity to spread influenza virus infection in wintertime (69). The transmission rate between infected and uninfected mice placed in the same cage was enhanced under 47% RH compared to 70% RH, as well as under lesser ventilated conditions. Because influenza transmission does not readily occur between infected and naïve mice simply placed in the same cage (67), mice experiments used mice-adapted influenza viruses (64, 65, 70).
In contrast to mice, guinea pigs allow transmission of human pathogenic influenza viruses and have some human-like properties such as the existence of the functional Mx GTPase antiviral gene and human type (α2-6) sialic acid receptor in the upper respiratory tract epithelia (68, 71). Due to these advantages, the effect of temperature and humidity on the transmission of influenza virus was investigated in guinea pigs (52, 53, 72). Four pairs of infected and uninfected guinea pigs were placed in climate chambers such that airflow was directed from the infected toward the uninfected guinea pigs. The transmission efficiency was evaluated by the virus shedding in the nasal wash from the exposed naïve animals. At 20°C, transmission of influenza virus was not observed under high RH (80%). In contrast, the transmission was highly efficient under low RH (∼20–35%) at 20°C. Viral transmission was generally more efficient at 5°C compared to 20°C. Of note, 5°C ambient air temperature allowed 50% transmission even under 80% RH.
One possible explanation of this result could be the reduced mucociliary clearance (MCC) and increased stability of virion remaining on the upper respiratory mucosa at 5°C (52). Another possible explanation is that AH at 5°C (∼5.5 g/m3) is much less than at 20°C (∼14 g/m3), although RH is the same (80%). In contrast to in temperate regions, respiratory infections have little seasonality in tropical regions. A study focusing on that aspect showed that no aerosol transmission was observed at 30°C at any humidity despite contact transmission being comparable at 30 and 20°C (53). Thus, high ambient temperature likely negates the effect of humidity on influenza transmission in tropical zones. Based on these results, Lowen & Palese (47) predict that aerosol transmission predominates during the winter season in temperate regions (because dry and warm indoor climate allows stability of influenza viruses in desiccated droplet nuclei that stay airborne for prolonged periods), while contact is the major mode of spread in the tropics (because in warm and humid climates, droplets evaporate less water and readily settle on surfaces). This hypothesis is illustrated in Table 1 and has considerable effect on proper precautions and public health measures against respiratory virus infections in different parts of the world and in different seasons.
More recently, ferrets have been used for evaluating the contribution of the environmental conditions to influenza viral transmission (54). In agreement with the results obtained in the previous studies using mice and guinea pigs, respiratory droplet transmission efficiency between ferrets was found to be most efficient under 23°C/30% RH conditions and least efficient at 23°C/50% RH and 5°C/70% RH. In addition to the finding that low RH enhances aerosol influenza transmission, there is another common thread found throughout the diverse animal models: that aerosol viral infection rate drops under intermediate RH atmosphere. Lowen et al. (52) observed that transmission between guinea pigs was inefficient at 50% RH and more efficient at both low (20–35%) and high (65%) RH at 20°C. Similarly, the transmission rate between ferrets at 30% and 70% RH was higher than that of 50% RH at 20°C (54). This phenomenon is consistent with the results demonstrated in the mouse aerosol infection model (70). In this study, the morbidity of mice exposed to virus-containing atmosphere under various RHs at ∼22–24°C was examined. At intermediate RH (∼40–60%), 77.5% of subjected mice survived, even though they were exposed to atomized virus suspension enough to kill all subjected mice at 23% RH. Therefore, an ideal humidity for preventing aerosol respiratory viral transmission at room temperature appears to be between 40% and 60% RH.
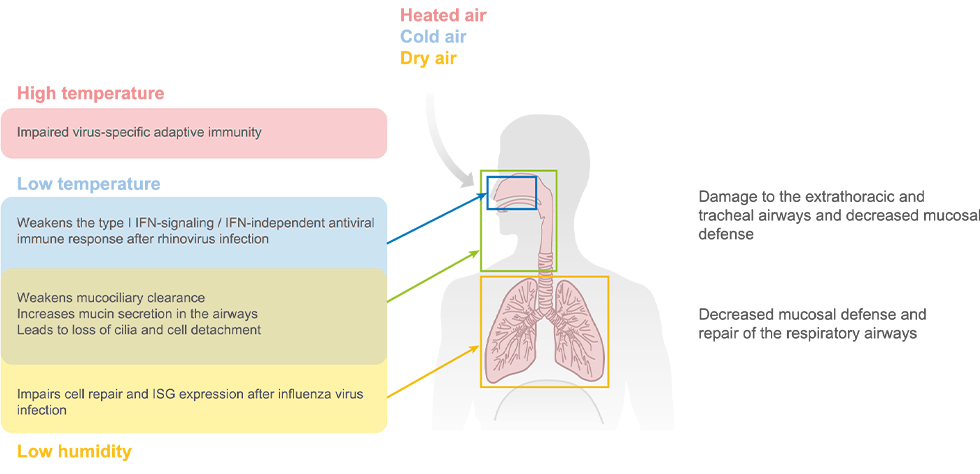
Figure 3
Effect of environmental factors on the host airway defense mechanisms. The extrathoracic and tracheal mucosal surface defense is directly affected by the seasonal changes in temperature and water content of the inhaled air on both infected and susceptible hosts. The immunological part of this effect extends into the lung periphery and lung tissue for unknown reasons. Abbreviations: IFN, interferon; ISG, interferon-stimulated gene. Figure adapted from image created with BioRender.com.
Effect of environmental factors on the host airway antiviral defense
The mucosal surface of the respiratory tract is continuously exposed to inhaled environmental air containing volatile and nonvolatile pollutants and potentially various pathogens. Multi-tiered host airway defense systems prevent infection by incoming respiratory viruses (73). Seasonal fluctuations of temperature and humidity of the inhaled air have been shown to directly affect the airway mucosal surface defense at multiple levels (Figure 3). In this section, we focus on the effect of environmental factors on the host airway antiviral defenses.
Intrinsic Barriers
The intrinsic barrier provides the first line of defense against respiratory viruses on the mucosal surface of the respiratory epithelium. Different airway epithelium composition in the different parts of the respiratory tract creates the airway diameter-dependent barrier defenses (73). The epithelial cells lining the airway surface comprise an efficient mechanical barrier, as well as provide MCC. Furthermore, mucus secreted from the goblet cells and submucosal glands in the larger conducting airways confers chemical barriers at the mucosal surface (74).
Mucus production
An incoming virus first must find epithelial cells to invade the host. Mucus layers can effectively trap the virus before it can enter the host cells (Figure 4). Mucus secreted from the submucosal glands within the lamina propria serves as a mechanical barrier and as a chemical barrier by its antimicrobial properties (74, 75). Components of the mucus are 93–97% w/w water, 3–7% w/w solids, 1–3% w/w glycoproteins, 1% w/w proteins, 0.5–1% w/w lipids, and 0.70–1.4% w/w minerals (76). The major glycoproteins in the airway mucus are secretory mucin proteins MUC5AC and MUC5B (77). Cold environments have been linked to exacerbations of chronic obstructive pulmonary disease (COPD), which manifests in chronic airflow obstruction, inflammation, and hypersecretion of respiratory mucus (78, 79).
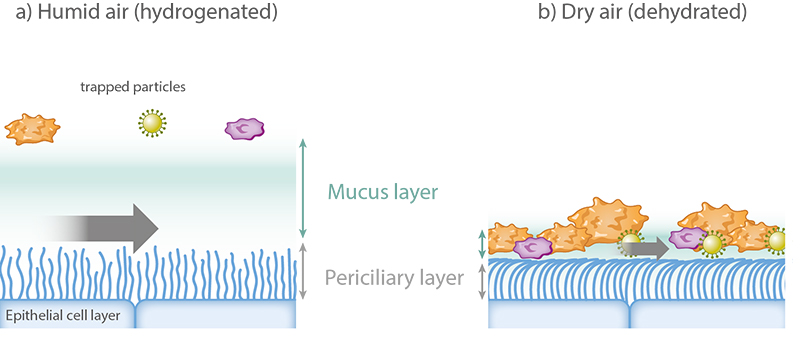
Figure 4
Effect of dry air on mucociliary clearance. (a) Proper mucus hydration is required for the efficient mucous transport. (b) Dehydration caused by dry breathing air leads to increased viscoelasticity of the mucous layer and immobilizes cilia, which are pressed down by the reduced height of the dehydrated periciliary layer. Figure adapted from image created with BioRender.com.
In the normal human bronchial epithelial (NHBE) cells isolated from COPD patients, the expression level of the transient receptor potential melastatin 8 (TRPM8), which is the cold receptor activated by temperature under 27°C or cooling agents including menthol (80, 81), is upregulated (82). Furthermore, cold (18°C) exposure or menthol treatment of cultured NHBE cells increases MUC5AC secretion in a TRPM8-dependent manner (82, 83). Another study examined the effect of temperature, humidity, and airflow mimicking respiration on mucin secretion from human nasal epithelial cells using a climate chamber for cell culture (84). Mucin production increased under 25°C, 40% RH compared to 37°C, 80% RH. Airflow increased mucin production under 25°C, 40% RH, but not at 37°C, 80% RH. These results suggest that impairment of MCC under low temperature and low humidity includes hypersecretion of mucin.
Airway epithelial integrity
The airways have a type I mucosal surface, which is covered by a single-layer epithelial lining to perform respiratory functions (85). The airway epithelial layer serves as the second line of defense after the mucus layer to provide a physical barrier within the respiratory tract. Immediate repair of the airway epithelia is critical to maintain the integrity of the respiratory tract. A study using guinea pigs demonstrates that the experimentally injured airway epithelial surface is re-established within 8–15 h (86). The epithelial cells at the edge of the damaged area migrate rapidly and flatten to cover the damaged zone, followed by re-epithelialization (86). Inhaling dry air causes immediate airway epithelial cilia loss, detachment of epithelial cells, and inflammation of the trachea of guinea pigs (87). Moreover, dry air exposure of mice impairs epithelial cell repair in the lung after influenza virus infection (88). Disruption of airway epithelial integrity caused by inhalation of dry air might be involved in the winter epidemics of certain types of respiratory virus infections.
Mucociliary clearance
MCC serves as a key mechanism for eliminating the inhaled pathogens and irritants from the respiratory epithelial surface (75). The double mucus layer with different viscosities enables efficient MCC (Figure 4).
The viscous mucosal layer facing the airway cavity entraps microparticles and microorganisms, and the watery lower mucus layer adjacent to the epithelia (periciliary layer) transmits the force of the ciliated cells to move microorganisms and particle-containing mucus toward the outside of the nose in mice and toward the larynx in humans, where mucus and entrapped particles and microbes are swallowed or expectorated (75). Inhaling cold air, which is always dry because of the limited water storage capacity of cold air, causes impairment of MCC. A study on the effect of ambient temperature on the ciliary beat frequency of the nasal and tracheal ciliated cells isolated from human subjects showed that mucociliary beating begins to decline as the temperature dips below 20°C and is no longer observed at 5°C (89). Another study focused on the MCC under various physiological conditions revealed that inhaling cold air slows MCC rates in living chickens (83). A more recent study showed that preincubation of mice in a low RH environment (10% RH) decreases MCC compared to 50% RH, resulting in impaired viral clearance following influenza virus infection (88). Given that the MCC depends on the maintenance of double mucus layers with two different viscosities and a delicate osmotic balance, proper mucus hydration is required for an efficient mucus transport. A review on the relationship between temperature and humidity of inhaled air and properties of airway mucosa found that 100% RH at core temperature is the optimal condition for the efficient mucosal functions and airway defense in humans (90). Mucus dehydration caused by breathing air of low humidity leads to decreased MCC. Water loss of the mucus layer transfers to the periciliary layer, reduces its height, and immobilizes the pressed down cilia (75) (Figure 4). The effect of humidity on nasal, tracheal, and bronchial MCC has been well studied in animals (88, 90, 91).
In humans, nasal MCC has been investigated (92–94). These studies showed that nasal MCC was not affected by dry breathing air in young healthy persons (92) but mucociliary speed decreased progressively in 174 test persons (different ages and genders) when RH of breathing air was reduced from 70% to 20% (93). One study showed that mucociliary speed is affected by alternative unilateral congestion and decongestion of the nasal cavities induced by the nasal cycle (94). Ventilation of anesthetized patients with unheated (<37°C) and not water-saturated anesthetic gases leads to diminished ciliary activity, cell damage, and ultimately cell death of bronchial epithelia (95, 96)
Inducible Antiviral Innate Immunity
Innate immune responses, induced in response to a viral infection, confer critical protection within the respiratory mucosa. Multiple classes of innate immune sensors recognize virus-associated molecular patterns to initiate downstream antiviral signaling, including the production of type I and type III IFNs (97). These IFNs are key effector cytokines that signal through their cognate receptors on neighboring cells to trigger the expression of hundreds of IFN-stimulated genes (ISGs). These ISGs act on various stages of the viral replication cycle to induce an antiviral state (98). Whether environmental factors affect the host antiviral innate immunity was previously unknown. Recent studies reveal that season-dependent environmental factors, such as temperature and humidity, can affect the host antiviral innate immunity against respiratory virus infections (88, 99–101).
Human rhinoviruses, a major cause of the common cold, cause illness mostly in winter. In tissue culture, rhinoviruses are known to replicate much better at 33°C, which mimics the cooler temperature of the nasal cavity, than at the core body temperature found in lower airways (37°C). What makes this virus so adept at replicating at the low temperature? A study focusing on the effect of ambient temperature on the host cells demonstrated that the preferential replication of rhinoviruses at 33°C has to do with the inefficient host antiviral response at this temperature (99). At 33°C, rhinoviruses triggered only low levels of type I IFN production from infected airway epithelial cells. Moreover, knocking out a key innate viral sensor signaling molecule, MAVS, needed to produce type I IFN from host cells rescued restricted rhinovirus replication at 37°C.
These results show that robust host antiviral response at the core body temperature might block rhinovirus spread in the lower airways due to robust host IFN response. Further, the study implies that exposure of the nose to cooler air during the winter may enable robust rhinovirus replication. In addition to the IFN production, a follow-up study revealed that both apoptosis and an antiviral ribonuclease, RNase L, confer temperature-dependent antiviral resistance to rhinovirus at the warmer temperature (100). These studies collectively suggest that effective rhinovirus replication in the lower temperature in the nasal cavity upon inhalation of cold air in the winter is driven in part by impaired host innate immune responses. Conversely, keeping the nose warm during the winter might boost antiviral innate resistance to the common cold virus.
One of the best-known links between an environmental factor and influenza disease is the drop in AH. Seasonal epidemics of influenza virus–related mortality are preceded by a drop in AH levels during the winter season in the United States (18). How does low outdoor AH affect seasonal influenza epidemics? As explained above, low outdoor AH leads to low indoor RH. A clue to this question comes from a recent study in mice that exposed mice to low RH of 10–20%. Using mice carrying a functional myxovirus resistance protein 1 (Mx1) gene, a key ISG that restricts influenza virus replication absent in most inbred mouse strains (102), the study found that Mx1 mice housed in 10–20% RH succumbed to influenza virus infection more rapidly than those housed in 50% RH. The study found at least three separate mechanisms that can contribute to the susceptibility of mice at low humidity. First, as discussed above, MCC was severely impaired at low humidity (88). Second, exposure to low humidity impaired airway tissue repair mechanisms. Third, a single-cell RNA sequencing analysis of the lung tissue collected from Mx1 mice revealed that the exposure to dry air impairs global ISG expressions following intranasal influenza virus infection (88). A striking finding is that ISG expression was impaired not only in the airway epithelial cells but also within the cell types found throughout the lung. How exactly dry air affects IFN response in the respiratory tract is currently unknown.
Other Innate Defense Mechanisms
Direct pathogen clearance by phagocytosis or production of reactive oxygen species (ROS) plays an important role as the nonspecific immune response. Seasonal oscillation of the daylight period modulates the physiological activity of mammalian species through daily melatonin pulse (9). Exposure of Siberian hamsters to the short daylight period (8 h) decreased phagocytotic activities and ROS productions of granulocytes and monocytes compared to the long daylight period (16 h) exposure (103). In contrast, the short daylight period increased natural killer cell cytotoxicity. Vitamin D biosynthesis is also modulated by sunlight. During the winter season, vitamin D deficiency is common presumably because of insufficient sunlight (10). In vitro cultures of bone marrow–derived macrophages isolated from vitamin D–deficient mice impair macrophage maturation, production of surface antigen as well as lysosomal enzymes, and H2O2 production (104). Collectively, these data suggest short daylight as a contributing factor in the impairment of the innate immune responses in winter.
Virus-Specific Adaptive Immunity
Adaptive immunity provides highly specific and long-lived protection against infectious agents. The initiation of adaptive immunity begins when antigen-presenting cells stimulate naïve virus-specific T cells to become activated, expand, and differentiate into effector T cells that can mediate antiviral responses at the site of infection (105). T follicular helper cells are also critical in promoting B cell activation and differentiation to provide antiviral antibody responses (106).
Apart from the respiratory virus infections, enhanced effector T cell–mediated responses in mice housed at a higher temperature have been described in the context of antitumor immunity (107, 108) as well as graft-versus-host disease (GVHD) (109). Housing mice at a higher temperature (30°C), a thermoneutral temperature for mice, suppressed tumor growth compared with the typical housing temperature at 22°C by increasing the number of antigen-specific CD8+ T cells (107). Consequently, sensitivity to the pancreatic cancer therapy was higher in mice housed at 30°C rather than 22°C (108). Similarly, transplantation of major histocompatibility complex–mismatched bone marrow cells induces severe GVHD in mice housed at 30°C, whereas those housed at 22°C are resistant to the onset of GVHD with the given treatment (109). These studies suggest that the housing temperature of the host can affect the adaptive immune responses in general and imply that vaccines should be given at an optimal temperature to induce maximal immunity.
In the context of respiratory virus infection, a recent study suggested that a high ambient temperature mimicking a summer heat wave weakens virus-specific adaptive immunity following influenza virus infection in mice (101). The study showed that heat exposure of mice (36°C) impairs virus-specific CD8+ T cell responses and antibody production after intranasal influenza virus infection. These impaired antiviral immune responses in heat-exposed mice were partially restored by glucose or short-chain fatty acid supplementation, suggesting a role for diet and microbiome in heat-mediated immune impairment. According to a Centers for Disease Control and Prevention surveillance report, all six recent influenza pandemics occurring in the Northern Hemisphere during 1957–2009 were spring to summer (110). The role of abnormal temperature fluctuations in flu pandemics will become even more relevant with the increasing effect of global warming and climate change.
Effect of environmental factors on disease tolerance to respiratory viruses
Disease tolerance is a mechanism to cope with infections by decreasing the deleterious effect of tissue damage caused by pathogens or host immune responses without directly affecting pathogen burden (111). For instance, lethal and pathological consequences of influenza infection in TLR7- and MAVS-deficient Mx1 congenic mice were tolerated in the absence of caspase-1/11 without affecting viral burden (102). This study revealed that lethality of influenza infection in the absence of innate resistance is mediated by the activation of inflammasome-mediated neutrophil activation
Low humidity exposure of Mx1 congenic mice has been shown to increase mortality, weight loss, and pulmonary viral burden following influenza virus infection (88). Furthermore, severe tissue damage after influenza virus infection was observed in the lung tissue of dry air–exposed mice. Of note, caspase-1/11 deficiency rescued disease and lethality that occurs in dry air–exposed infected mice. These studies suggest that mice exposed to low humidity conditions, which dampen global ISG expressions and impair antiviral resistance, can tolerate the infection if they are lacking the inflammasome caspases. This concept can be extended to other settings in which antiviral innate resistance is impaired, such as in older adults (112). Thus, interfering with inflammasome caspases might provide a therapeutic window to counter the deleterious consequences of influenza-mediated diseases by enhancing disease tolerance.
Concluding remarks and future directions
By virtue of being exposed to ambient air by breathing, the nasal and tracheal mucosal surface of the respiratory tract is affected by ambient temperature and the water content of the inhaled air (74). Inhalation of dry air causes epithelial damage, MCC impairment, and increased mucin production (84, 87, 88). Impaired ISG expression and tissue repair and increased viral burden and mortality after influenza virus infection have been proven in mice exposed to 7 days of low RH of 10–20% (88). Devastating disease course following dry air exposure is mediated by inflammasome caspase activation. Similarly, inhalation of cold ambient air impairs MCC and increases mucin production (82, 83, 89). Lower temperature impairs type I IFN-mediated and IFN-independent antiviral defense mechanisms after rhinovirus infection (99, 100). In contrast, exposure of mice to heat wave–level high temperature dampens virus-specific adaptive immune responses after influenza virus infection (101). The animal studies demonstrate a dramatic effect of environmental conditions on every aspect of host response to respiratory infection and disease.
The intervention studies in school and nursery children, office workers, and army recruits have shown that increasing humidity from low to median range reduced respiratory infection rates and absenteeism (113). The human-to-human transmission of the SARS-CoV-2 in Wuhan, China, began in December 2019 (4, 5). SARS-CoV-2 is a close relative of SARS-CoV (114), which spread during the winter of 2002–2003 (6, 7). Given that the expression of the receptor for both SARS-CoV-2 and SARS-CoV, angiotensin-converting enzyme 2 (114, 115), appears to be concentrated in a small population of type II alveolar cells (116), we speculate that the low humidity and temperature environment would promote the viability of SARS-CoV-2 in the droplets and impaired ciliary clearance and innate immune defense, for robust access to the deep lung tissue and rapid transmission between infected individuals. Since the respiratory airways, where the type I and II alveolar cells are located, are not reachable by respiratory droplets with a diameter of more than 5 micrometers (45, 46), it appears likely that at least the severe cases of COVID-19 with viral pneumonia are the result of airborne transmission events. A recent study that examined province-level variability of the basic reproductive numbers of COVID-19 across China found that not only dry and cold locations experience high viral spread, but certain locations with high AH also have higher viral transmission within the population (117). The precise relationship between temperature, humidity, and COVID-19 will become more evident as the Northern Hemisphere reaches the summer months.
Seasonal changes in the environmental factors can affect not only local defense mechanisms but also systemic physiological changes. Thermoneutral temperature housing potentiates antitumor immunity and GVHD onset in mice (107–109). In addition, a short daylight period and consequent deficiency of vitamin D impair nonspecific immune responses (103, 104). In conclusion, the combination of low humidity, temperature, and sunlight may trigger an impairment of the local and systemic antiviral defense mechanisms, leading to the increased host susceptibility to the respiratory viruses in winter (Figure 5).
A number of studies demonstrate the effect of the environmental factors on the respiratory virus stability and transmission rates. In addition, several studies now reveal the effects of environmental factors on the host defense to the respiratory virus infection and the underlying molecular mechanisms. Collectively, we can begin to assemble the factors that promote viral spread and disease in the winter months for cold and influenza viruses. However, other respiratory virus infections peak in spring or summer. One of the possible explanations is the replication conflict among respiratory viruses. Co-infection of winter respiratory viruses and spring respiratory viruses in the animal model may provide insights into the unknown mechanisms of spring-to-summer epidemics. Another unresolved issue is the observation that even in the highly controlled environment of the animal housing setting (22.2°C, 50% RH, controlled light/dark cycle) using the same viral stock, rate of transmission of influenza virus was higher when the experiments were carried out in the winter (November to April, 58.2%) versus summer (May to October, 34.1%) (65). Thus, in addition to temperature, lighting, and humidity, there may be other environmental factors controlled by the seasons that contribute to higher levels of influenza virus infections in the winter months.
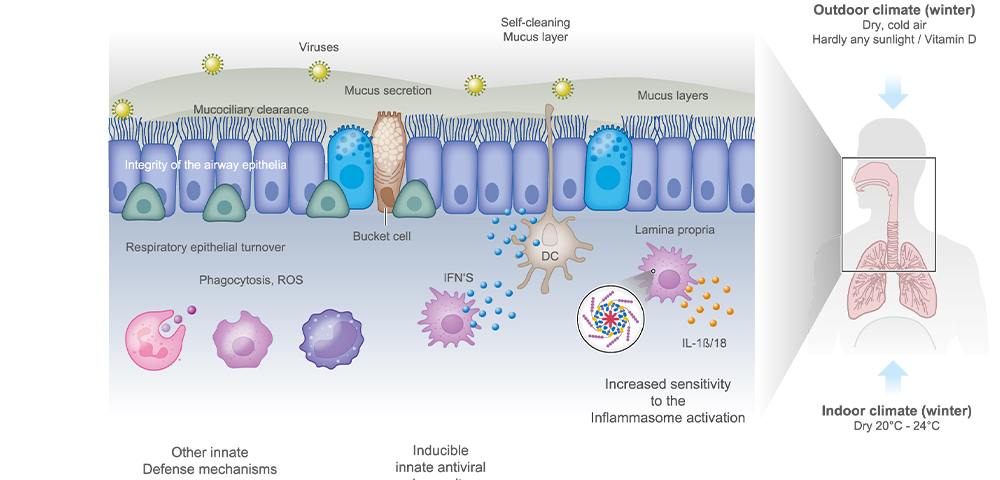
Figure 5
Possible mechanisms of increased host susceptibility to respiratory virus infections in winter. Inhalation of cold dry air directly affects the upper airway mucosa, impairs mucociliary clearance, and increases mucin production. In addition, inhalation of dry air per se causes epithelial damage. A short daylight period and consequent deficiency of vitamin D impair direct pathogen clearance. Cold and dry air impairs local antiviral innate immune responses after viral infection. Abbreviations: DC, dendritic cell; IFN, interferon; ROS, reactive oxygen species. Figure adapted from image created with BioRender.com.
How might we use these insights to prevent respiratory infections and illnesses in the winter months? In addition to vaccines and antiviral drugs, nonpharmaceutical interventions to prevent respiratory infections are gaining attention. Lifestyle (eating healthy, sleeping more than 7 h/day) and hygiene practices (washing hands, wearing face masks) are known to increase antimicrobial resistance and prevent transmission, respectively (118–121). In addition to these measures, we might consider controlling the indoor environment to combat respiratory infections. Such interventions with humidifiers have been realized since the 1960s with promising results (122–127). More recently, a study in Minnesota found that humidifying preschool classrooms during January to March to ∼45% RH results in a significant reduction in the total number of influenza virus and viral genome copies found in the air and on objects compared to control classrooms (128). Such nonpharmaceutical interventions can be combined with vaccination strategies to achieve better prevention of respiratory viral infections (Table 2).
| Tips |
Related reference(s) |
| Humidification of indoor air to maintain humidity to 40–60% relative humidity at room temperature |
47, 49, 52, 70, 113, 128 |
| Ventilation of indoor air |
69 |
| Wearing face mask to keep the nose warm and moist |
88, 90, 93, 99, 100 |
| Vitamin D supplement to compensate for short daylight–induced vitamin D deficiency |
103, 104, 118, 120 |
| Sleeping more than 7 h/day |
119 |
| Washing hands to prevent indirect contact transmission |
120, 121 |
TABEL 2
Tips for limiting respiratory virus transmission in winter
| Stability / infectivity of aerosolized "winter viruses", "summer viruses" and "year-round viruses"
|
TABEL 3
Non-rhinovirus enteroviruses are “summer viruses”, with the exception of norovirus. The stability of other enteroviruses other than polioviruses is unknown
red color marks the RF, in which the stability / infectivity is maintained for a maximum of long

 Figure 1
Figure 1 Figure 2
Figure 2 Figure 3
Figure 3 Figure 4
Figure 4 Figure 5
Figure 5 TABEL 3
TABEL 3