Study by Edward W. Dunklin and Theodore T. Puck
Methods
The experiments were carried out in the 640 cubic foot chambers previously described (7) which allow precise control of temperature and relative humidity over fairly wide ranges.
Seven and a half hour cultures of microorganisms were grown in heart infusion broth (Difco) containing added serum and dextrose, and suspensions prepared from these cultures were atomized into the experimental chambers for 1 minute by the standard technique already described (7). The bacterial suspensions were dispersed by reflux type atomizers (8) whose particle size distribution was measured by means of a cascade impactor
1 (9).
By careful standardization of the spraying procedure it was found possible to introduce a fairly constant number of microorganisms into the 640 cubic foot chamber in each experiment. This number was fixed at about 5 million. Daily checks on the total number of microorganisms discharged by the atomizer were carried out by repeating the spraying procedure with the atomizer connected directly to a bubbler sampler (10), so that the entire spray was absorbed in the bubbler fluid, aliquots of which were then plated in nutrient agar. The bacterial content of the air was determined over a 2 hour period after atomization of the microorganisms, by means of successive 2 minute bubbler samples and by exposure of agar settling plates (7).
Time of trial (interval between the end of the spraying process and the start of sampling)
in minutes |
Number of pneumococci per cubic foot of air obtained with the bubbler sampler |
Number of pneumococci on a tray set up for 5 minutes |
| 0 |
5 200 |
332 |
| 5 |
4 580 |
290 |
| 10 |
4 000 |
247 |
| 20 |
3 800 |
193 |
| 30 |
3 240 |
177 |
| 45 |
2 700 |
152 |
| 60 |
2 420 |
106 |
| 75 |
2 240 |
96 |
| 90 |
1 940 |
90 |
| 105 |
1 800 |
74 |
| 120 |
1 620 |
66 |
TABLE I
Survival of pneumococci, Type I, Sprayed from Broth Suspension into Air at a Temperature of 22.2°C. and a Rdative Humidity of 19 Per Cent
Mass median diameter of the bacterial spray emergent from the atomizer was 3.2 µm.
The survival curves obtained by these two sampling methods paralleled each other very closely. By and large, the bubbler sampler values were somewhat more uniform, and so these have been used for calculations of the survival constants which will be reported here. A typical experimental protocol is shown in Table I.
The relative humidity was varied over a range extending from 3 to 80 per cent at three different temperatures, 14.4, 22.2 and 33.3°C. For those regions of temperature and humidity where very high bacterial death rates were encountered, the results were checked by many repetitions of each experiment.
Test results
The most notable result of these investigations was the demonstration of the existence of a narrow range of relative humidity close to 50 percent, which is rapidly lethal to bacteria freshly sprayed into the air from a suspension in the culture medium.
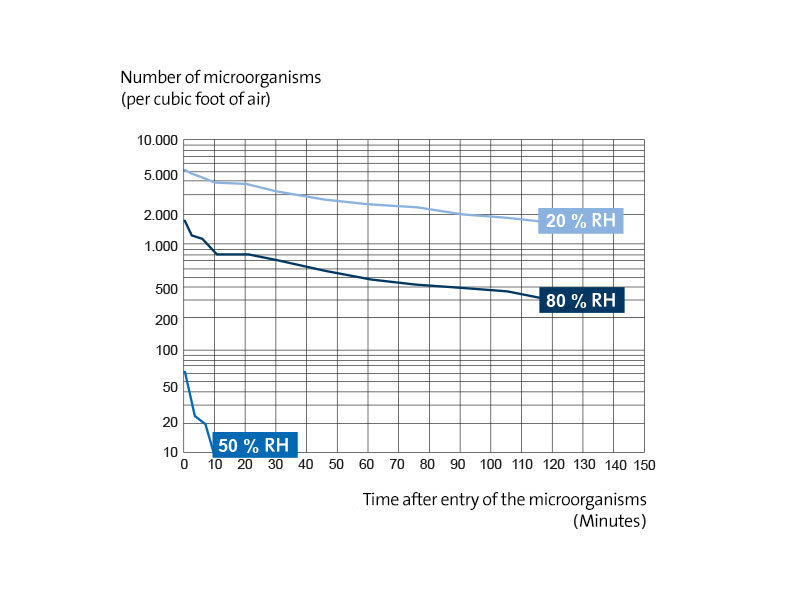
The pneumococcus, a relatively sensitive microorganism, was found to be the most susceptible to this lethal effect of the atmosphere, while the effect was far less pronounced in the group C streptococcus and the staphylococcus. Fig. 1 shows typical results of an experiment with pneumococci at three different relative humidities, which demonstrate the ability of the microorganism to survive over long periods of time at both very low and very high relative humidities, but not at medium values.
Figure 1
Logarithmic representation of the survival of pneumococci sprayed into the atmosphere from nutrient culture at various relative humidities and 22.2 ° C. The cloud emerging from the atomizer had a median mass diameter of 3.2 µm. Approximately equal numbers of microorganisms were introduced into the chamber in each experiment.
Analysis of the survival curves shows that at least two independent lethal processes are involved that affect the rate of death. The first process always takes place very quickly and causes pronounced death between the 5 minutes and 20 minutes after the droplets with microorganisms have been introduced into the air. The second decay process proceeds more slowly and generally extends over the entire two-hour observation period (Fig. 1).
Both dying processes result in linear logarithmic survival curves, so that when the number of surviving germs is plotted as a logarithmic function of time, the resulting curve consists of two straight lines that intersect at a point between 5 and 20 minutes after the microorganisms have been introduced into the chamber. It was determined that the slopes of these two parts of the curve depend on the relative humidity and temperature, the nature of the microorganisms used, the composition of the liquid medium in which they are suspended and the particle size of the droplets within which they are released into the atmosphere be introduced, be influenced. Experimental effects demonstrating the mode of action of these factors have been studied most extensively for pneumococci.
Experiments comparable to those shown in Fig. 1 were always carried out using suspensions of seven and a half hour pneumococcal cultures in a serum dextrose medium, at many different relative humidities. For each experiment, the number of surviving germs was plotted as a logarithmic function of time (as shown in Fig. 1) and values for the two constants K
1 und K
2 (which is the logarithmic rates of disappearance of viable microorganisms from the air during the first 10 to 15 minutes (K
1) or for the following interval (K>sub>2) represent) were determined at each relative humidity at 22.2 ° C. The nebulizer used in this process creates a cloud of droplets of bacteria suspended in the nutrient medium, their median mass diameter
2 beim Austritt aus dem Zerstäuber 3,2 µm beträgt.
Fig. 2 presents a graph of these survival curves. It can be seen that in a narrow range of relative humidity between 40 and 55 percent, the death rate of the microorganisms sprayed into the air from the suspension in the nutrient medium is greatly accelerated.
Figure 2
Slopes of the logarithmic survival curves for pneumococci which were sprayed from suspension in the nutrient medium in atmospheres of different relative humidity. K
1 is the slope of the first part of the survival curve, K
2 is the logarithmic decay constant for the last part of the curve. The value for K
1 at 50 percent relative humidity, 0.32 ± 0.12, is the mean of the results from more than a dozen experiments. Exact values for K
2 in the middle humidity range could not be determined because only a few microorganisms survived longer than 20 minutes.
Effects of the type of microorganism
Staphylococcus albus and Streptococcus hemolyticus group C showed logarithmic survival curves that were comparable to those shown in Fig. 1 for pneumococcus.
The effects of relative humidity on the death rates of these microorganisms followed the same pattern. The only differences observed were the relative extent of the effect and a slight shift in the relative humidity value with the maximum death rate. The order of increasing susceptibility to the fatal effect of a mean relative humidity was: hemolytic streptococcus group C, staphylococcus and pneumococcus type I. The effect of different relative humidities on the survival constants of streptococcus and staphylococcus is shown in Fig. 3.
Figure 3
Logarithmic decay constants K
1 and K
2 of haemolytic streptococcus group C and of Staphylococcus albus as a function of the relative humidity. The microorganisms were sprayed into the air from the suspension in the nutrient medium.
Effect of weaning
It is necessary to determine to what extent the disappearance of the microorganisms from the air represents a real process of death, as opposed to the removal of airborne particles by settling or coalescence. To investigate this, a series of tests with the conditions presented in Fig. 2 was repeated with a microbial nutrient culture to which methylene blue was added. Samples of the chamber air were taken at the same time intervals as in the previous experiments and through a solution of 70% alcohol in H
2O bubbled. The total amount of accumulated dye was then determined photometrically for each of these samples. In this way, the rate of removal of these particles from the air by inelastic collisions with the walls and floor of the chamber was determined.
Figure 4
Determination of the settling rates by measuring the persistence of droplets with microorganisms and dye in the air at different relative humidities. The suspension medium was a nutrient solution and the immediate median mass diameter of the cloud was 3.2 µm. (The ordinate units are arbitrary, the figures only show the relative amounts of dye per cubic foot of air as a function of time.)
Fig. 4 shows exemplary representations of this data. The logarithmic curves determined in this way also consist of two straight lines with an incision, usually between 10 and 20 minutes. However, if the slopes of the curves are plotted against the entire spectrum of relative humidity, as in Fig. 5, then neither for K
1 still for K
2 to observe a maximum, as was the case with the extraction of surviving microorganisms from the air. The sedimentation rate remains low at all relative humidities
3, while the rate of disappearance of viable pneumococci, for example, can be increased by 50 to 100 fold at medium humidity. Since the rapid disappearance of microorganisms that were sprayed from suspension in the nutrient medium in atmospheres of medium humidity cannot be explained by settling or collision processes, an actual lethal process must take place at relative humidity around 50 percent.
4
An explanation for this rapid death of airborne germs, which occurs in atmospheres with medium relative humidity, must be made by analyzing the processes that take place when droplets loaded with bacteria are introduced into unsaturated air. The evaporation of water from such droplets initially results in an increase in the concentration of all soluble substances in the liquid that surround the microorganism. Once enough water has been lost, one or more dissolved substances can reach a concentration that is toxic to the cell. At the same time, however, the bacterial cell itself also loses water.
When the cell dries out severely, it becomes resistant to many types of physical and chemical stressors (11) and thus gains a certain immunity to the destructive effects of highly concentrated solutes.
Figure 5
Gradients of the sedimentation curves of droplets from a 3.2 µm cloud, introduced in atmospheres with different relative humidities. K
1 is the slope of the first part of the curve and K.
2 the logarithmic decay constant for the last segment. The difference between this curve and that from Fig. 2 shows that the increased rate of disappearance of viable microorganisms from the air at medium relative humidities cannot be explained by a settling process.
Figure 6
FIG. 6. Logarithmic survival constants of pneumococci, from a suspension in distilled H
2O sprayed into the air as a function of relative humidity.
How much water such a cell loses depends on the relative humidity and the binding energy between water and the various components of the cell. At very low humidity values, the water removal is so pronounced that a stable state can be achieved in which the microorganism is not impaired by the high concentrations of dissolved substances.
Conversely, when the relative humidity is high, sufficient water is bound to and around the cell in order to avoid the development of lethal conditions. The experiments described here indicate that at medium humidity values, the amount of water that remains directly associated with the microorganism causes a critical condition in the cell. In this condition, the bacterium becomes very susceptible to the toxic effects of the unphysiological concentrations of solutes remaining from the original medium in which the cells were suspended.
Effect of different compositions of the suspension liquid
In view of the previous evaluation, it can be assumed that the increase in the death rate at medium values of relative humidity would be greatly reduced if the microorganisms were sprayed into the air from a suspension in distilled water instead of nutrient solution. This conclusion has been verified experimentally. Seven and a half hour cultures of type I pneumococcus were centrifuged, once in distilled H
2O washed and resuspended in water before being sprayed into the experimental chamber. The survival rate was determined in the usual way. The characteristic linear logarithmic survival curves with a sharp incision after the first 10 or 15 minutes were observed exactly as in the experiments with nutrient solution. In contrast to the latter studies, however, the substitution of nutrient solution with distilled water ensured a complete elimination of the sharp increase in the death rate at humidity levels of around 50 percent. Fig. 6 shows the variation of the logarithmic survival constants of pneumococci, which were sprayed into the air from suspension in distilled water, with the relative humidity. A comparison of the curves from Fig. 5 and 6 shows that the survival pattern of these airborne pneumococci is practically identical to the persistence of an aerosol of colored droplets, which indicates the absence of lethal processes.
The question arose as to which of the components of the nutrient solution exerted the toxic effects that were observed at relative humidity around 50 percent. To answer them, separate solutions of the various components of the nutrient solution were prepared. The solution of each component was prepared in the same concentration as that in the standard nutrient solution and used as a suspension medium from which pneumococci were sprayed into the air. These experiments showed that NaCl, present in the original solution in a concentration of 0.5 percent, was largely responsible for the lethal effect. None of the other components of the nutrient solution, whether tested individually or in combination, could cause a lethal effect at medium relative humidity. The determination of the survival constants of pneumococcal cultures which were sprayed into the air from a suspension with all components except NaCl5 resulted in a pair of curves comparable to that in Figure 6.
If, on the other hand, a solution with only 0.5 percent NaCl was used, the survival curves were as in Figure 2. The characteristic rapid increase in the mortality rate at mean relative humidity therefore only occurs in the presence of NaCl.
With these results in mind, it was important to study the effect of relative humidity on bacteria sprayed into the air from a suspension in saliva. This fluid represents the natural medium from which respiratory bacteria are introduced into the ambient air, so that the survival pattern of the microorganisms suspended in it can be directly linked to the problems of natural airborne infections. Saliva was collected from human volunteers who had previously chewed paraffin for a few minutes to stimulate salivation. No attempt was made to rid the fluid of its normal bacterial population, as these microorganisms were present in the final suspension in such small numbers relative to the pneumococci that they represented a negligible contamination. A seven and a half hour culture of type I pneumococci was centrifuged and resuspended in an identical volume of saliva. The resulting suspension was immediately transferred to the nebulizer and sprayed into the chamber using the usual procedure as described in the previous experiments. The resulting survival pattern was comparable to that obtained when using nutrient or saline solution (Fig. 2) as the suspension medium, but not with the curve that resulted from the use of distilled water (Fig. 6).
Effect of the droplet size
If the death of bacteria sprayed into the air from a suspension in the nutrient medium or saliva is actually related to a dehydration process, then a relative humidity, which is normally lethal, should cause this effect to a much weaker degree if the bacteria are suspended in small droplets will be held in larger ones. This conclusion can be drawn because the smaller droplet also contains a smaller amount of the harmful substances and thus the microorganism is exposed to a lower concentration as dehydration progresses. This effect was investigated by comparing the survival rates of pneumococci, which were sprayed into the air from a suspension in serum dextrose nutrient solution, using two different nebulizers, the particle size distributions of which corresponded to median mass diameters of 1.6 µm and 3.2 µm, respectively .
Fig. 7 shows a typical series of tests at 22.2 ° C and 50 percent relative humidity, which demonstrates that the death rate is higher for pneumococci suspended in larger droplets. In a series of more than 15 experiments at 22.2 ° C, the average value of the decay constant was K.
1, which is observed during the first 10 to 20 minutes after the end of the spraying process, for particles of 1.6 µm diameter at 0.012 ± 0.005 per minute and for particles of 3.2 µm diameter at 0.32 ± 0.12 per minute. The average value for K
2, the death rate in the second section of the survival curve, was 0.045 per minute for the 1.6 µm particles. (For the particles of 3.2 µm, only so few bacteria had survived after the first 20 minutes that a satisfactory value for K
2 could be determined at 50 percent relative humidity.)
Figure 7
Effect of the particle size in the cloud on the survival of pneumococci, which are sprayed from the suspension in the nutrient solution in an atmosphere of 50 percent relative humidity at 22.2 ° C.
Figure 8
Effect of temperature on the survival constants of pneumococci which are sprayed from the suspension in the nutrient solution in atmospheres with different relative humidities.
Influence of temperature
The effects of temperature changes on this lethal process have also been studied. The survival of pneumococci from suspensions in the nutrient solution sprayed into the air at various values of relative humidity was measured at 14.4 ° C and at 33.3 ° C and at 22.2 ° C.
As expected, a pronounced temperature coefficient was observed; K
1 varied by a factor of fifteen between these temperature extremes. In Fig. 8 are the values of K
1 and K
2 shown as a function of the relative humidity at the three temperatures. As before, the values for K
2 in the case of medium humidity levels, inaccurate for the two higher temperatures, since the extremely high initial death rates under these conditions mean that too few surviving organisms remain for an investigation of the subsequent lethal process.
Discussion
When a suspension of a given composition is atomized into the atmosphere, the ultimate degree of dehydration will depend on the relative humidity as the droplet will eventually enter a state of equilibrium with the atmosphere. When this occurs, the particle has a water content that creates a surface tension that is identical to the partial pressure of water in the air. At low relative humidity, a particle that contains a microorganism will have lost almost all of its water, some of which could even have been in permanent chemical association with certain cellular components. At high relative humidity, on the other hand, only loosely bound water is retained. At medium relative humidity, partial dehydration of the various cellular systems occurs. Calculations show that spherical droplets of distilled water can evaporate from an original diameter of 3 µm to a tenth of this size within 0.004 seconds, even at a relative humidity of 50 percent.6 This is significantly faster than any of the death rates observed in the experiments presented here so that the vaporization process would be complete before the lethal process could even begin. Accordingly, the mortality rates observed in these experiments must be interpreted as a representation of the natural mortality of the microorganisms in a given chemical environment, at a degree of hydration that is in equilibrium with the prevailing relative humidity.
If the theoretical formulation proposed here is correct, the salt activity is not solely responsible for the lethal effect observed at medium humidity. Droplets of pure sodium chloride solution sprayed into the air are saturated with this substance at a relative humidity of 75 percent (13) and would dry out completely at lower humidity. In our experiments, however, a maximum lethal effect was shown for microorganisms suspended in pure saline solution at relative humidity of 50 percent, not 75 percent. This apparent discrepancy can be explained by the fact that the salt in a bacterial suspension goes into solution with some cellular components and forms compounds. The maximum lethal effect of the salt bound in the cell unfolds at the relative humidity that dries the microorganism to the point that it is most vulnerable, not at a humidity in which there is an equilibrium with a saturated solution of pure NaCl.
The present investigation results were explained by the postulation of the existence of a critical moisture content, at which a bacterial cell becomes much more susceptible to toxic substances than it would be if the water content was higher or lower. Further evidence suggests that this phenomenon is characteristic of bacterial systems. This principle is the basis of the lyophilization process for drying viable microorganisms. If water is withdrawn from bacterial cells at room temperature, the culture dies very quickly. To avoid this, the bacteria are first cooled to a temperature that is so low that the rate of the lethal process is greatly reduced. Then the water is withdrawn as quickly as possible. After the cells have been dehydrated enough to leave the critical area, they can be brought back to room temperature without showing any pronounced mortality. Applying these considerations has enabled the development of a simple method of drying microorganisms without resorting to the low temperatures used in the lyophilization process. It has been shown that cells can be dehydrated at room temperature without impairing their survivability, as long as the drying takes place so quickly that the stable state is reached before the lethal processes can take effect. These studies will be described in a forthcoming publication (14). The much greater efficiency of steam sterilization compared to heating processes in dry conditions is undoubtedly based on the same phenomenon.
Microorganisms heated in the presence of steam remain in a state of moderate hydration, making them more susceptible to the lethal effects of the high temperatures than they would be in a fully desiccated state. Another example of how this effect works can be found in the reduced effectiveness of the elimination of airborne germs by aerosol-type disinfectants such as glycols at low relative humidity (5, 6). This effect is due in part to the fact that the germicidal vapor does not condense as well on dried particles that arise when a bacterial cloud is dispersed in very dry ambient air. However, recent in vitro experiments have also shown that completely dried bacteria are more resistant to the lethal effects of glycols in the liquid state (15).
The values for K
1 und K
2as calculated here, represent the logarithmic rates of the removal of viable bacteria from the atmosphere and thus contain both the settling rates and the lethal processes due to the presence of NaCl at critical humidity values. If the corresponding settling rates from Fig. 5 are subtracted from these, the mortality rates of all three microorganisms examined here are extremely small, both at very low and at very high relative humidity levels. In some cases the result is negative, which simply means that the death rate is too low for this technique to accurately evaluate.
The lethal effect emanating from NaCl at a critical level of cellular dehydration is probably due, among other things, to the denaturation of one or more vital enzyme systems. Further studies of the mechanism of this lethal process and the behavior of the bacterial cells in different hydration states are in progress.
Further studies are required to evaluate the significance of the results reported here for epidemiological problems. This will require more detailed studies of the behavior of a larger number of pathogens, particularly in the virus group. Possibly these effects can also help explain some aspects of the seasonal prevalence of some respiratory diseases. It is also possible that the moisture regulation itself can have a certain effect as a prophylactic measure. Preliminary experiments have shown that by introducing a carefully controlled amount of steam into the air of a chamber, an air-bound dispersion of pneumococci that have been dried out for an hour in a very dry atmosphere can be rehydrated and rapidly killed. Although increasing the relative humidity above 50 percent cannot reduce airborne germ contamination as effectively as glycol vapor or UV radiation, the simplicity of this measure would recommend its use in a wide variety of situations, if only partially beneficial.
Summary
The viability of type I pneumococcus sprayed into the atmosphere from a liquid suspension was measured as a function of relative humidity. When using a nutrient solution, saliva or 0.5 percent saline solution as the suspension medium, very high mortality rates have been observed at relative humidity around 50 percent. However, if the air humidity is above or below this value, the microorganisms survive for longer periods of time.
By measuring the settling rate of the droplets used in these experiments, it was demonstrated that the disappearance of the microorganisms from the air was indeed a lethal process, not just a manifestation of collision processes. If a salt-free solution is used, the clear peak death rate observed at medium relative humidity disappears. The lethal effect of mean relative humidity on pneumococci atomized from a saline suspension increases when the particle size of the atomized droplets is increased or when the temperature is increased.
Cultures of hemolytic streptococcus group C and staphylococcus, also nebulized from a nutrient medium, show the same general pattern of survival as a function of relative humidity, although the mortality rates are lower than for pneumococcus.
These effects can be explained by assuming the existence of a critical level of cellular dehydration, at which the microorganisms become much more susceptible to toxic substances than they are when more or less water is bound to the cell.
The results presented here could be essential in explaining certain aspects of the epidemiology of droplet infection.
1The same nebulizer was used for all of the experiments described here, except for those cases where the effect of different particle sizes was investigated.
2That is, 50 percent of the mass of the cloud was contained in droplets with a diameter of less than 3.2 µm.
3It is particularly low when the relative humidity is below 12 percent. The sharp incision in the curve at this point would mean that the particles are completely dehumidified at this relative humidity.
4These weaning data also suggest that the faster rate of disappearance of a bacterial suspension during the first 10 to 20 minutes after nebulization (i.e. the difference between K.
1 and K
2) is caused by processes that selectively affect larger particles more than smaller ones. The settling of the larger particles out of the cloud in the first 10 to 15 minutes is undoubtedly responsible for the increased initial rate of disappearance of the colored aerosol. The same process, combined with any influence the larger particle size per se might have on accelerating the death of bacteria contained in the droplets, would prove the existence of two separate death rates, K
1 and K
2which are typically observed when a cloud of droplets containing bacteria is introduced into the atmosphere under the conditions described here (see below, section on particle size).
5That is, this solution contained 1 percent peptone, 1 percent tryptose, 0.05 percent dextrose, and 2.0 percent serum.
6 As has already been shown (12), the evaporation rate of small droplets in unsaturated air corresponds to the following equation: dA/dt= 8πDM/RTd(ρ_g-〖ρ'〗_g)
where A = area of the droplet, D = vapor diffusion coefficient, for water vapor in the air at room temperature equals 0.24 cm
2 per second; M = molecular weight of vapor, 18.0 for H.
20; R = gas constant, 62 400 cc. x mm. Hg. Mol
-1 deg.
-1; T = absolute temperature, 296 ° A; d = density of the liquefied vapor, which is the unit for water; ρ
g = Partial pressure of water vapor in the atmosphere, which would be the product of the relative humidity and the vapor pressure of water at the temperature of the experiment; and ρ '
g = Pressure of the water vapor in equilibrium with the droplet.
For droplets of pure water, ρ ‘would correspond to the pure vapor pressure, which at 22.2 ° C is 20.1 mm Hg. If the droplet contains other substances dissolved in the aqueous phase, ρ ‘would be reduced by the proportion that corresponds to the reduction in vapor pressure caused by the combination of the dissolved substances. The presence of salts and proteins in the droplets would reduce the rate of evaporation somewhat, but not even a saturated saline solution could reduce the vapor pressure to an extent sufficient to reduce the rate of evaporation significantly.
This work was supported by the United States Public Health Service, the Bartlett Memorial Fund of the University of Chicago, and the Carbide and Carbon Chemicals Corporation.
Submitted to the Graduate School of the University of Chicago in partial fulfillment of the requirements for the degree Master of Science (Edward W. Dunklin).